Artificial Soils Reveal Individual Factor Controls on Microbial Processes
- PMID: 35880897
- PMCID: PMC9426496
- DOI: 10.1128/msystems.00301-22
Artificial Soils Reveal Individual Factor Controls on Microbial Processes
Abstract
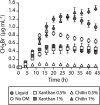
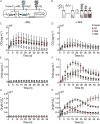
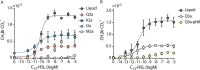
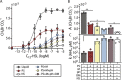
Soil matrix properties influence microbial behaviors that underlie nutrient cycling, greenhouse gas production, and soil formation. However, the dynamic and heterogeneous nature of soils makes it challenging to untangle the effects of different matrix properties on microbial behaviors. To address this challenge, we developed a tunable artificial soil recipe and used these materials to study the abiotic mechanisms driving soil microbial growth and communication. When we used standardized matrices with varying textures to culture gas-reporting biosensors, we found that a Gram-negative bacterium (Escherichia coli) grew best in synthetic silt soils, remaining active over a wide range of soil matric potentials, while a Gram-positive bacterium (Bacillus subtilis) preferred sandy soils, sporulating at low water potentials. Soil texture, mineralogy, and alkalinity all attenuated the bioavailability of an acyl-homoserine lactone (AHL) signaling molecule that controls community-level microbial behaviors. Texture controlled the timing of AHL sensing, while AHL bioavailability was decreased ~105-fold by mineralogy and ~103-fold by alkalinity. Finally, we built artificial soils with a range of complexities that converge on the properties of one Mollisol. As artificial soil complexity increased to more closely resemble the Mollisol, microbial behaviors approached those occurring in the natural soil, with the notable exception of organic matter. IMPORTANCE Understanding environmental controls on soil microbes is difficult because many abiotic parameters vary simultaneously and uncontrollably when different natural soils are compared, preventing mechanistic determination of any individual soil parameter's effect on microbial behaviors. We describe how soil texture, mineralogy, pH, and organic matter content can be varied individually within artificial soils to study their effects on soil microbes. Using microbial biosensors that report by producing a rare indicator gas, we identify soil properties that control microbial growth and attenuate the bioavailability of a diffusible chemical used to control community-level behaviors. We find that artificial soils differentially affect signal bioavailability and the growth of Gram-negative (Escherichia coli) and Gram-positive (Bacillus subtilis) microbes. These artificial soils are useful for studying the mechanisms that underlie soil controls on microbial fitness, signaling, and gene transfer.
Keywords: acylhomoserine lactone; artificial soils; biosensor; cell signaling; indicator gas; soil; synthetic biology; water retention curve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cavicchioli R, Ripple WJ, Timmis KN, Azam F, Bakken LR, Baylis M, Behrenfeld MJ, Boetius A, Boyd PW, Classen AT, Crowther TW, Danovaro R, Foreman CM, Huisman J, Hutchins DA, Jansson JK, Karl DM, Koskella B, Mark Welch DB, Martiny JBH, Moran MA, Orphan VJ, Reay DS, Remais JV, Rich VI, Singh BK, Stein LY, Stewart FJ, Sullivan MB, van Oppen MJH, Weaver SC, Webb EA, Webster NS. 2019. Scientists’ warning to humanity: microorganisms and climate change. Nat Rev Microbiol 17:569–586. doi:10.1038/s41579-019-0222-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
