Prehospital Lyophilized Plasma Transfusion for Trauma-Induced Coagulopathy in Patients at Risk for Hemorrhagic Shock: A Randomized Clinical Trial
- PMID: 35881397
- PMCID: PMC9327575
- DOI: 10.1001/jamanetworkopen.2022.23619
Prehospital Lyophilized Plasma Transfusion for Trauma-Induced Coagulopathy in Patients at Risk for Hemorrhagic Shock: A Randomized Clinical Trial
Abstract
Importance: Blood transfusion is a mainstay of therapy for trauma-induced coagulopathy, but the optimal modalities for plasma transfusion in the prehospital setting remain to be defined.
Objective: To determine whether lyophilized plasma transfusion can reduce the incidence of trauma-induced coagulopathy compared with standard care consisting of normal saline infusion.
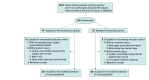
Design, setting, and participants: This randomized clinical trial was performed at multiple centers in France involving prehospital medical teams. Participants included 150 adults with trauma who were at risk for hemorrhagic shock and associated coagulopathy between April 1, 2016, and September 30, 2019, with a 28-day follow-up. Data were analyzed from November 1, 2019, to July 1, 2020.
Intervention: Patients were randomized in a 1:1 ratio to receive either plasma or standard care with normal saline infusion (control).
Main outcomes and measures: The primary outcome was the international normalized ratio (INR) on arrival at the hospital. Secondary outcomes included the need for massive transfusion and 30-day survival. As a safety outcome, prespecified adverse events included thrombosis, transfusion-related acute lung injury, and transfusion-associated circulatory overload.
Results: Among 150 randomized patients, 134 were included in the analysis (median age, 34 [IQR, 26-49] years; 110 men [82.1%]), with 68 in the plasma group and 66 in the control group. Median INR values were 1.21 (IQR, 1.12-1.49) in the plasma group and 1.20 (IQR, 1.10-1.39) in the control group (median difference, -0.01 [IQR, -0.09 to 0.08]; P = .88). The groups did not differ significantly in the need for massive transfusion (7 [10.3%] vs 4 [6.1%]; relative risk, 1.78 [95% CI, 0.42-8.68]; P = .37) or 30-day survival (hazard ratio for death, 1.07 [95% CI, 0.44-2.61]; P = .89). In the full intention-to-treat population (n = 150), the groups did not differ in the rates of any of the prespecified adverse events.
Conclusions and relevance: In this randomized clinical trial including severely injured patients at risk for hemorrhagic shock and associated coagulopathy, prehospital transfusion of lyophilized plasma was not associated with significant differences in INR values vs standard care with normal saline infusion. Nevertheless, these findings show that lyophilized plasma transfusion is a feasible and safe procedure for this patient population.
Trial registration: ClinicalTrials.gov Identifier: NCT02736812.
Conflict of interest statement
Figures
References
-
- Crowe EP, Goel R, Ness PM. Prothrombin and partial thromboplastin time. In: Moore HB, Neal MD, Moore EE, eds. Trauma Induced Coagulopathy. Springer International Publishing; 2021:265-270.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

