A complete biomimetic iron-sulfur cubane redox series
- PMID: 35881795
- PMCID: PMC9351461
- DOI: 10.1073/pnas.2122677119
A complete biomimetic iron-sulfur cubane redox series
Abstract
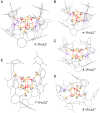
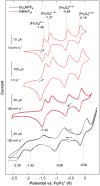
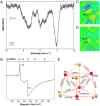
Synthetic iron-sulfur cubanes are models for biological cofactors, which are essential to delineate oxidation states in the more complex enzymatic systems. However, a complete series of [Fe4S4]n complexes spanning all redox states accessible by 1-electron transformations of the individual iron atoms (n = 0-4+) has never been prepared, deterring the methodical comparison of structure and spectroscopic signature. Here, we demonstrate that the use of a bulky arylthiolate ligand promoting the encapsulation of alkali-metal cations in the vicinity of the cubane enables the synthesis of such a series. Characterization by EPR, 57Fe Mössbauer spectroscopy, UV-visible electronic absorption, variable-temperature X-ray diffraction analysis, and cyclic voltammetry reveals key trends for the geometry of the Fe4S4 core as well as for the Mössbauer isomer shift, which both correlate systematically with oxidation state. Furthermore, we confirm the S = 4 electronic ground state of the most reduced member of the series, [Fe4S4]0, and provide electrochemical evidence that it is accessible within 0.82 V from the [Fe4S4]2+ state, highlighting its relevance as a mimic of the nitrogenase iron protein cluster.
Keywords: Mössbauer spectroscopy; all-ferrous cubane; electrochemistry; iron-sulfur clusters; nitrogenase.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Burgess B. K., Lowe D. J., Mechanism of molybdenum nitrogenase. Chem. Rev. 96, 2983–3012 (1996). - PubMed
-
- Boncella A. E., et al. , The expanding utility of iron-sulfur clusters: Their functional roles in biology, synthetic small molecules, maquettes and artificial proteins, biomimetic materials, and therapeutic strategies. Coord. Chem. Rev. 453, 214229 (2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

