Label-free viability assay using in-line holographic video microscopy
- PMID: 35882977
- PMCID: PMC9325748
- DOI: 10.1038/s41598-022-17098-y
Label-free viability assay using in-line holographic video microscopy
Abstract
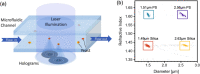
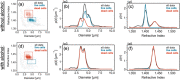
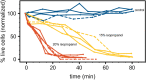
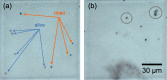
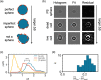
Total holographic characterization (THC) is presented here as an efficient, automated, label-free method of accurately identifying cell viability. THC is a single-particle characterization technology that determines the size and index of refraction of individual particles using the Lorenz-Mie theory of light scattering. Although assessment of cell viability is a challenge in many applications, including biologics manufacturing, traditional approaches often include unreliable labeling with dyes and/or time consuming methods of manually counting cells. In this work we measured the viability of Saccharomyces cerevisiae yeast in the presence of various concentrations of isopropanol as a function of time. All THC measurements were performed in the native environment of the sample with no dilution or addition of labels. Holographic measurements were made with an in-line holographic microscope using a 40[Formula: see text] objective lens with plane wave illumination. We compared our results with THC to manual counting of living and dead cells as distinguished with trypan blue dye. Our findings demonstrate that THC can effectively distinguish living and dead yeast cells by the index of refraction of individual cells.
© 2022. The Author(s).
Conflict of interest statement
All of the authors are employed by Spheryx, Inc. the manufacturer of xSight and xCells which were used to perform the research reported in this publication.
Figures
References
-
- Pray L. LH Hartwell’s yeast: A model organism for studying somatic mutations and cancer. Nat. Educ. 2008;1:183.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

