Effectiveness of aromatherapy for prevention or treatment of disease, medical or preclinical conditions, and injury: protocol for a systematic review and meta-analysis
- PMID: 35883155
- PMCID: PMC9317467
- DOI: 10.1186/s13643-022-02015-1
Effectiveness of aromatherapy for prevention or treatment of disease, medical or preclinical conditions, and injury: protocol for a systematic review and meta-analysis
Abstract
Background: Aromatherapy - the therapeutic use of essential oils from plants (flowers, herbs or trees) to treat ill health and promote physical, emotional and spiritual well-being - is one of the most widely used natural therapies reported by consumers in Western countries. The Australian Government Department of Health (via the National Health and Medical Research Council) has commissioned a suite of independent evidence evaluations to inform the 2019-20 Review of the Australian Government Rebate on Private Health Insurance for Natural Therapies. This protocol is for one of the evaluations: a systematic review that aims to examine the effectiveness of aromatherapy in preventing and/or treating injury, disease, medical conditions or preclinical conditions.
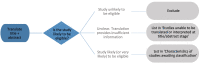
Methods: Eligibility criteria: randomised trials comparing (1) aromatherapy (delivered by any mode) to no aromatherapy (inactive controls), (2) aromatherapy (delivered by massage) to massage alone or (3) aromatherapy to 'gold standard' treatments.
Populations: any condition, pre-condition, injury or risk factor (excluding healthy participants without clearly identified risk factors).
Outcomes: any for which aromatherapy is indicated. Searches: Cochrane Central Register of Controlled Trials (CENTRAL), with a supplementary search of PubMed (covering a 6-month lag period for processing records in CENTRAL and records not indexed in MEDLINE), AMED and Emcare. No date, language or geographic limitations will be applied.
Data and analysis: screening by two authors, independently (records indexed by Aromatherapy or Oils volatile or aromatherapy in title; all full text) or one author (remaining records) with second author until 80% agreement. Data extraction and risk of bias assessment (ROB 2.0) will be piloted by three authors, then completed by a single author and checked by a second. Comparisons will be based on broad outcome categories (e.g. pain, emotional functioning, sleep disruption) stratified by population subgroups (e.g. chronic pain conditions, cancer, dementia) as defined in the analytic framework for the review. Meta-analysis or other synthesis methods will be used to combine results across studies. GRADE methods will be used to assess certainty of evidence and summarise findings.
Discussion: Results of the systematic review will provide a comprehensive and up-to-date synthesis of evidence about the effectiveness of aromatherapy.
Systematic review registration: PROSPERO CRD42021268244.
Keywords: Aromatherapy; CAM; Complementary and alternative medicine; Complementary medicine; Essential oil therapy; Integrative medicine; Massage; Meta-analysis; Natural therapies; Supportive therapy; Systematic review; Volatile oils.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- National Health and Medical Research Council . Statement of requirement: evidence evaluations for review of natural therapies (tranche two) 2020.
-
- National Health and Medical Research Council. Aromatherapy description developed in conversation with the National Health and Medical Research Council’s Natural Therapies Working Committee Chair and the Department of Health’s Natural Therapies Review Expert Advisory Panel (February 2020). 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

