Self-adjuvanting cancer nanovaccines
- PMID: 35883176
- PMCID: PMC9316869
- DOI: 10.1186/s12951-022-01545-z
Self-adjuvanting cancer nanovaccines
Abstract
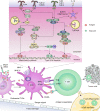
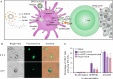
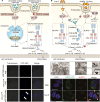
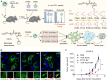
Nanovaccines, a new generation of vaccines that use nanoparticles as carriers and/or adjuvants, have been widely used in the prevention and treatment of various diseases, including cancer. Nanovaccines have sparked considerable interest in cancer therapy due to a variety of advantages, including improved access to lymph nodes (LN), optimal packing and presentation of antigens, and induction of a persistent anti-tumor immune response. As a delivery system for cancer vaccines, various types of nanoparticles have been designed to facilitate the delivery of antigens and adjuvants to lymphoid organs and antigen-presenting cells (APCs). Particularly, some types of nanoparticles are able to confer an immune-enhancing capability and can themselves be utilized for adjuvant-like effect for vaccines, suggesting a direction for a better use of nanomaterials and the optimization of cancer vaccines. However, this role of nanoparticles in vaccines has not been well studied. To further elucidate the role of self-adjuvanting nanovaccines in cancer therapy, we review the mechanisms of antitumor vaccine adjuvants with respect to nanovaccines with self-adjuvanting properties, including enhancing cross-presentation, targeting signaling pathways, biomimicking of the natural invasion process of pathogens, and further unknown mechanisms. We surveyed self-adjuvanting cancer nanovaccines in clinical research and discussed their advantages and challenges. In this review, we classified self-adjuvanting cancer nanovaccines according to the underlying immunomodulatory mechanism, which may provide mechanistic insights into the design of nanovaccines in the future.
Keywords: Antigen presentation; Cancer immunotherapy; Lymph node; Nanovaccine; Self-adjuvanting.
© 2022. The Author(s).
Conflict of interest statement
JFL holds interest in POP Biotechnologies. Other authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

