Beneficial Shifts in the Gut Bacterial Community of Gilthead Seabream (Sparus aurata) Juveniles Supplemented with Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO)
- PMID: 35883368
- PMCID: PMC9312144
- DOI: 10.3390/ani12141821
Beneficial Shifts in the Gut Bacterial Community of Gilthead Seabream (Sparus aurata) Juveniles Supplemented with Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO)
Abstract
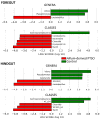
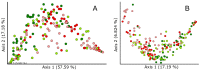
This study analyzes the potential use of an Allium-derived compound, propyl propane thiosulfonate (PTSO), as a functional feed additive in aquaculture. Gilthead seabream (Sparus aurata) juveniles had their diet supplemented with this Allium-derived compound (150 mg/kg of PTSO) and were compared with control fish. The effects of this organosulfur compound were tested by measuring the body weight and analyzing the gut microbiota after 12 weeks. The relative abundance of potentially pathogenic Vibrio and Pseudomonas in the foregut and hindgut of supplemented fish significantly decreased, while potentially beneficial Lactobacillus increased compared to in the control fish. Shannon's alpha diversity index significantly increased in both gut regions of fish fed with a PTSO-supplemented diet. Regarding beta diversity, significant differences between treatments only appeared in the hindgut when minority ASVs were taken into account. No differences occurred in body weight during the experiment. These results indicate that supplementing the diet with Allium-derived PTSO produced beneficial changes in the intestinal microbiota while maintaining the productive parameters of gilthead seabream juveniles.
Keywords: Allium-based phytogenic; Sparus aurata; body weight; gilthead seabream; gut microbiota; propyl propane thiosulfonate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies.Molecules. 2022 Oct 14;27(20):6900. doi: 10.3390/molecules27206900. Molecules. 2022. PMID: 36296491 Free PMC article.
-
Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Reduces Vibrio Populations and Increases Body Weight of European Seabass (Dicentrarchus labrax) Juveniles.Antibiotics (Basel). 2023 Jan 10;12(1):134. doi: 10.3390/antibiotics12010134. Antibiotics (Basel). 2023. PMID: 36671335 Free PMC article.
-
Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Attenuates Metabolic Alterations in Mice Fed a High-Fat Diet through Its Anti-Inflammatory and Prebiotic Properties.Nutrients. 2021 Jul 28;13(8):2595. doi: 10.3390/nu13082595. Nutrients. 2021. PMID: 34444755 Free PMC article.
-
Risk assessment and environmental consequences of the use of the Allium-derived compound propyl-propane thiosulfonate (PTSO) in agrifood applications.Environ Res. 2023 Nov 1;236(Pt 1):116682. doi: 10.1016/j.envres.2023.116682. Epub 2023 Jul 15. Environ Res. 2023. PMID: 37459943
-
In Vitro Antitumor and Anti-Inflammatory Activities of Allium-Derived Compounds Propyl Propane Thiosulfonate (PTSO) and Propyl Propane Thiosulfinate (PTS).Nutrients. 2023 Mar 11;15(6):1363. doi: 10.3390/nu15061363. Nutrients. 2023. PMID: 36986093 Free PMC article.
Cited by
-
Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies.Molecules. 2022 Oct 14;27(20):6900. doi: 10.3390/molecules27206900. Molecules. 2022. PMID: 36296491 Free PMC article.
-
Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Reduces Vibrio Populations and Increases Body Weight of European Seabass (Dicentrarchus labrax) Juveniles.Antibiotics (Basel). 2023 Jan 10;12(1):134. doi: 10.3390/antibiotics12010134. Antibiotics (Basel). 2023. PMID: 36671335 Free PMC article.
-
Microalgae and phytase dietary supplementation improved growth and gut microbiota in juvenile European seabass (Dicentrarchus labrax).BMC Genomics. 2024 Sep 6;25(1):838. doi: 10.1186/s12864-024-10760-x. BMC Genomics. 2024. PMID: 39242559 Free PMC article.
-
Enhancing growth performance in Liangshan black sheep through fermented onion: insights from transcriptomics and metabolomics.Front Vet Sci. 2025 May 15;12:1533728. doi: 10.3389/fvets.2025.1533728. eCollection 2025. Front Vet Sci. 2025. PMID: 40444105 Free PMC article.
-
The Impact of Tank Disinfectants on the Development of Microbiota in Gilthead Seabream (Sparus aurata) Larviculture Systems.Microorganisms. 2025 Jun 11;13(6):1359. doi: 10.3390/microorganisms13061359. Microorganisms. 2025. PMID: 40572247 Free PMC article.
References
-
- FAO . Sustainability in Action. FAO; Rome, Italy: 2020. The State of World Fisheries and Aquaculture 2020; pp. 1–224. - DOI
-
- Yukgehnaish K., Kumar P., Sivachandran P., Marimuthu K., Arshad A., Paray B.A., Arockiaraj J. Gut microbiota metagenomics in aquaculture: Factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 2020;12:1903–1927. doi: 10.1111/raq.12416. - DOI
-
- Dawood M.A.O., Koshio S., Esteban M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018;10:950–974. doi: 10.1111/raq.12209. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

