Development of a Biotechnology Platform for the Fast-Growing Cyanobacterium Synechococcus sp. PCC 11901
- PMID: 35883428
- PMCID: PMC9313322
- DOI: 10.3390/biom12070872
Development of a Biotechnology Platform for the Fast-Growing Cyanobacterium Synechococcus sp. PCC 11901
Abstract
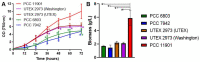
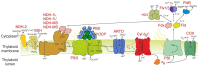
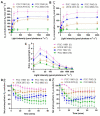
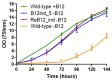
Synechococcus sp. PCC 11901 reportedly demonstrates the highest, most sustained growth of any known cyanobacterium under optimized conditions. Due to its recent discovery, our knowledge of its biology, including the factors underlying sustained, fast growth, is limited. Furthermore, tools specific for genetic manipulation of PCC 11901 are not established. Here, we demonstrate that PCC 11901 shows faster growth than other model cyanobacteria, including the fast-growing species Synechococcuselongatus UTEX 2973, under optimal growth conditions for UTEX 2973. Comparative genomics between PCC 11901 and Synechocystis sp. PCC 6803 reveal conservation of most metabolic pathways but PCC 11901 has a simplified electron transport chain and reduced light harvesting complex. This may underlie its superior light use, reduced photoinhibition, and higher photosynthetic and respiratory rates. To aid biotechnology applications, we developed a vitamin B12 auxotrophic mutant but were unable to generate unmarked knockouts using two negative selectable markers, suggesting that recombinase- or CRISPR-based approaches may be required for repeated genetic manipulation. Overall, this study establishes PCC 11901 as one of the most promising species currently available for cyanobacterial biotechnology and provides a useful set of bioinformatics tools and strains for advancing this field, in addition to insights into the factors underlying its fast growth phenotype.
Keywords: CodA selection; SacB selection; Synechococcus sp. PCC 11901; cellular metabolism; comparative genomics; photoinhibition; photosynthesis; vitamin B12.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lea-Smith D.J., Howe C.J. The Use of Cyanobacteria for Biofuel Production. In: Love J., Bryant J.A., editors. Biofuels and Bioenergy. Wiley; New York, NY, USA: 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

