Biotechnological Approaches to Optimize the Production of Amaryllidaceae Alkaloids
- PMID: 35883449
- PMCID: PMC9313318
- DOI: 10.3390/biom12070893
Biotechnological Approaches to Optimize the Production of Amaryllidaceae Alkaloids
Abstract
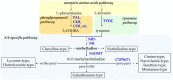
Amaryllidaceae alkaloids (AAs) are plant specialized metabolites with therapeutic properties exclusively produced by the Amaryllidaceae plant family. The two most studied representatives of the family are galanthamine, an acetylcholinesterase inhibitor used as a treatment of Alzheimer's disease, and lycorine, displaying potent in vitro and in vivo cytotoxic and antiviral properties. Unfortunately, the variable level of AAs' production in planta restricts most of the pharmaceutical applications. Several biotechnological alternatives, such as in vitro culture or synthetic biology, are being developed to enhance the production and fulfil the increasing demand for these AAs plant-derived drugs. In this review, current biotechnological approaches to produce different types of bioactive AAs are discussed.
Keywords: amaryllidaceae alkaloids; bioactive molecules; biosynthesis; biotechnological approach; in vitro cultures; synthetic biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Martin S.F. The Alkaloids: Chemistry and Pharmacology. Volume 30. Elsevier; Cambridge, MA, USA: 1987. The amaryllidaceae alkaloids; pp. 251–376.
-
- He M., Qu C., Gao O., Hu X., Hong X. Biological and pharmacological activities of amaryllidaceae alkaloids. RSC Adv. 2015;5:16562–16574. doi: 10.1039/C4RA14666B. - DOI
-
- Desgagné-Penix I. Biosynthesis of alkaloids in Amaryllidaceae plants: A review. Phytochem. Rev. 2020;20:409–431. doi: 10.1007/s11101-020-09678-5. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

