Getting Closer to Decrypting the Phase Transitions of Bacterial Biomolecules
- PMID: 35883463
- PMCID: PMC9312465
- DOI: 10.3390/biom12070907
Getting Closer to Decrypting the Phase Transitions of Bacterial Biomolecules
Abstract
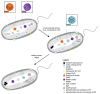
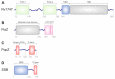
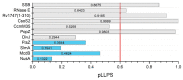
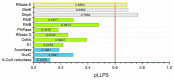
Liquid-liquid phase separation (LLPS) of biomolecules has emerged as a new paradigm in cell biology, and the process is one proposed mechanism for the formation of membraneless organelles (MLOs). Bacterial cells have only recently drawn strong interest in terms of studies on both liquid-to-liquid and liquid-to-solid phase transitions. It seems that these processes drive the formation of prokaryotic cellular condensates that resemble eukaryotic MLOs. In this review, we present an overview of the key microbial biomolecules that undergo LLPS, as well as the formation and organization of biomacromolecular condensates within the intracellular space. We also discuss the current challenges in investigating bacterial biomacromolecular condensates. Additionally, we highlight a summary of recent knowledge about the participation of bacterial biomolecules in a phase transition and provide some new in silico analyses that can be helpful for further investigations.
Keywords: bacterial cells; biomacromolecular condensates; liquid–liquid phase separation; membraneless organelles; multivalent interactions; phase transitions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Protein phase separation and its role in chromatin organization and diseases.Biomed Pharmacother. 2021 Jun;138:111520. doi: 10.1016/j.biopha.2021.111520. Epub 2021 Mar 23. Biomed Pharmacother. 2021. PMID: 33765580 Review.
-
Liquid-liquid phase separation in biology: mechanisms, physiological functions and human diseases.Sci China Life Sci. 2020 Jul;63(7):953-985. doi: 10.1007/s11427-020-1702-x. Epub 2020 Apr 30. Sci China Life Sci. 2020. PMID: 32548680 Review.
-
Protein Databases Related to Liquid-Liquid Phase Separation.Int J Mol Sci. 2020 Sep 16;21(18):6796. doi: 10.3390/ijms21186796. Int J Mol Sci. 2020. PMID: 32947964 Free PMC article. Review.
-
Biological Liquid-Liquid Phase Separation, Biomolecular Condensates, and Membraneless Organelles: Now You See Me, Now You Don't.Int J Mol Sci. 2023 Aug 24;24(17):13150. doi: 10.3390/ijms241713150. Int J Mol Sci. 2023. PMID: 37685957 Free PMC article.
-
Reorganization of Cell Compartmentalization Induced by Stress.Biomolecules. 2022 Oct 8;12(10):1441. doi: 10.3390/biom12101441. Biomolecules. 2022. PMID: 36291650 Free PMC article. Review.
Cited by
-
Bacterial nucleoid is a riddle wrapped in a mystery inside an enigma.J Bacteriol. 2024 Mar 21;206(3):e0021123. doi: 10.1128/jb.00211-23. Epub 2024 Feb 15. J Bacteriol. 2024. PMID: 38358278 Free PMC article. Review.
-
Sequence-Based Prediction of Protein Phase Separation: The Role of Beta-Pairing Propensity.Biomolecules. 2022 Nov 28;12(12):1771. doi: 10.3390/biom12121771. Biomolecules. 2022. PMID: 36551199 Free PMC article.
-
The Role of Intrinsically Disordered Proteins in Liquid-Liquid Phase Separation during Calcium Carbonate Biomineralization.Biomolecules. 2022 Sep 9;12(9):1266. doi: 10.3390/biom12091266. Biomolecules. 2022. PMID: 36139105 Free PMC article. Review.
-
Liquid-Liquid Phase Separation and Protective Protein Aggregates in Bacteria.Molecules. 2023 Sep 12;28(18):6582. doi: 10.3390/molecules28186582. Molecules. 2023. PMID: 37764358 Free PMC article. Review.
References
-
- Brangwynne C.P., Tompa P., Pappu R.V. Polymer physics of intracellular phase transitions. Nat. Phys. 2015;11:899–904. doi: 10.1038/nphys3532. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

