Acute Effects of Focused Ultrasound-Induced Blood-Brain Barrier Opening on Anti-Pyroglu3 Abeta Antibody Delivery and Immune Responses
- PMID: 35883506
- PMCID: PMC9313174
- DOI: 10.3390/biom12070951
Acute Effects of Focused Ultrasound-Induced Blood-Brain Barrier Opening on Anti-Pyroglu3 Abeta Antibody Delivery and Immune Responses
Abstract
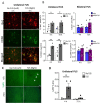
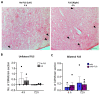
Alzheimer's Disease (AD) is a neurodegenerative disorder characterized by the accumulation of amyloid plaques and hyperphosphorylated tau in the brain. Currently, therapeutic agents targeting amyloid appear promising for AD, however, delivery to the CNS is limited due to the blood-brain-barrier (BBB). Focused ultrasound (FUS) is a method to induce a temporary opening of the BBB to enhance the delivery of therapeutic agents to the CNS. In this study, we evaluated the acute effects of FUS and whether the use of FUS-induced BBB opening enhances the delivery of 07/2a mAb, an anti-pyroglutamate-3 Aβ antibody, in aged 24 mo-old APP/PS1dE9 transgenic mice. FUS was performed either unilaterally or bilaterally with mAb infusion and the short-term effect was analyzed 4 h and 72 h post-treatment. Quantitative analysis by ELISA showed a 5-6-fold increase in 07/2a mAb levels in the brain at both time points and an increased brain-to-blood ratio of the antibody. Immunohistochemistry demonstrated an increase in IgG2a mAb detection particularly in the cortex, enhanced immunoreactivity of resident Iba1+ and phagocytic CD68+ microglial cells, and a transient increase in the infiltration of Ly6G+ immune cells. Cerebral microbleeds were not altered in the unilaterally or bilaterally sonicated hemispheres. Overall, this study shows the potential of FUS therapy for the enhanced delivery of CNS therapeutics.
Keywords: focused ultrasound; microglia; pyroglutamate-3 Aβ.
Conflict of interest statement
Stephan Schilling is an advisor to Vivoryon Therapeutics N.V. Co-senior author, Cynthia A. Lemere, was previously an unpaid scientific advisory board member at Probiodrug AG, (currently Vivoryon Therapeutics N.V.), receives antibodies from Vivoryon as a gift-in-kind, and has previously received unrestricted funding from Probiodrug AG for a pGlu3 Aβ immunotherapy study. She serves as a consultant to Biogen and Acumen Pharmaceuticals, both of which are developing anti-amyloid immunotherapies. Brigham and Women’s Hospital holds two patents related to the FUS work.
Figures


Similar articles
-
Focused ultrasound with anti-pGlu3 Aβ enhances efficacy in Alzheimer's disease-like mice via recruitment of peripheral immune cells.J Control Release. 2021 Aug 10;336:443-456. doi: 10.1016/j.jconrel.2021.06.037. Epub 2021 Jun 26. J Control Release. 2021. PMID: 34186148 Free PMC article.
-
Focused Ultrasound-Induced Blood-Brain Barrier Opening Enhances GSK-3 Inhibitor Delivery for Amyloid-Beta Plaque Reduction.Sci Rep. 2018 Aug 27;8(1):12882. doi: 10.1038/s41598-018-31071-8. Sci Rep. 2018. PMID: 30150769 Free PMC article.
-
Focused ultrasound-mediated blood-brain barrier opening in Alzheimer's disease: long-term safety, imaging, and cognitive outcomes.J Neurosurg. 2022 Nov 4;139(1):275-283. doi: 10.3171/2022.9.JNS221565. Print 2023 Jul 1. J Neurosurg. 2022. PMID: 36334289
-
"Focused Ultrasound-mediated Drug Delivery in Humans - a Path Towards Translation in Neurodegenerative Diseases".Pharm Res. 2022 Mar;39(3):427-439. doi: 10.1007/s11095-022-03185-2. Epub 2022 Mar 7. Pharm Res. 2022. PMID: 35257286 Free PMC article. Review.
-
Efficacy and safety of focused ultrasound-mediated blood-brain barrier opening in Alzheimer's disease: A systematic review and meta-analysis.J Alzheimers Dis Rep. 2025 May 21;9:25424823251343789. doi: 10.1177/25424823251343789. eCollection 2025 Jan-Dec. J Alzheimers Dis Rep. 2025. PMID: 40406679 Free PMC article. Review.
Cited by
-
Immunity in neuromodulation: probing neural and immune pathways in brain disorders.J Neuroinflammation. 2025 Apr 28;22(1):122. doi: 10.1186/s12974-025-03440-4. J Neuroinflammation. 2025. PMID: 40296049 Free PMC article. Review.
-
Safety, Efficacy and Clinical Applications of Focused Ultrasound-Mediated Blood Brain Barrier Opening in Alzheimer's Disease: A Systematic Review.J Prev Alzheimers Dis. 2024;11(4):975-982. doi: 10.14283/jpad.2024.85. J Prev Alzheimers Dis. 2024. PMID: 39044508 Free PMC article.
-
Brain Endothelial Cells in Blood-Brain Barrier Regulation and Neurological Therapy.Int J Mol Sci. 2025 Jun 18;26(12):5843. doi: 10.3390/ijms26125843. Int J Mol Sci. 2025. PMID: 40565303 Free PMC article. Review.
-
Temporal Characterization of the Amyloidogenic APPswe/PS1dE9;hAPOE4 Mouse Model of Alzheimer's Disease.Int J Mol Sci. 2024 May 25;25(11):5754. doi: 10.3390/ijms25115754. Int J Mol Sci. 2024. PMID: 38891941 Free PMC article.
-
Using focused ultrasound to modulate microglial structure and function.Front Cell Neurosci. 2023 Dec 18;17:1290628. doi: 10.3389/fncel.2023.1290628. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38164436 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

