Cell Confluence Modulates TRPV4 Channel Activity in Response to Hypoxia
- PMID: 35883510
- PMCID: PMC9313184
- DOI: 10.3390/biom12070954
Cell Confluence Modulates TRPV4 Channel Activity in Response to Hypoxia
Abstract
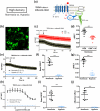
Transient receptor potential vanilloid 4 (TRPV4) is a polymodal Ca2+-permeable channel involved in various hypoxia-sensitive pathophysiological phenomena. Different tools are available to study channel activity, requiring cells to be cultured at specific optimal densities. In the present study, we examined if cell density may influence the effect of hypoxia on TRPV4 activity. Transiently TRPV4-transfected HEK293T cells were seeded at low or high densities corresponding to non-confluent or confluent cells, respectively, on the day of experiments, and cultured under in vitro normoxia or hypoxia. TRPV4-mediated cytosolic Ca2+ responses, single-channel currents, and Ca2+ influx through the channel were measured using Ca2+ imaging/microspectrofluorimetric assay, patch-clamp, and Bioluminescence Resonance Energy Transfer (BRET), respectively. TRPV4 plasma membrane translocation was studied using confocal microscopy, biotinylation of cell surface proteins, and BRET. Our results show that hypoxia exposure has a differential effect on TRPV4 activation depending on cell confluence. At low confluence levels, TRPV4 response is increased in hypoxia, whereas at high confluence levels, TRPV4 response is strongly inhibited, due to channel internalization. Thus, cell density appears to be a crucial parameter for TRPV4 channel activity.
Keywords: BRET; TRP channel; calcium; cell culture conditions; cell density; patch-clamp; stretch-activated channel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

