Therapeutic Potential of a Novel Vitamin D3 Oxime Analogue, VD1-6, with CYP24A1 Enzyme Inhibitory Activity and Negligible Vitamin D Receptor Binding
- PMID: 35883516
- PMCID: PMC9312876
- DOI: 10.3390/biom12070960
Therapeutic Potential of a Novel Vitamin D3 Oxime Analogue, VD1-6, with CYP24A1 Enzyme Inhibitory Activity and Negligible Vitamin D Receptor Binding
Abstract
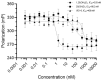
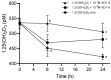
The regulation of vitamin D3 actions in humans occurs mainly through the Cytochrome P450 24-hydroxylase (CYP24A1) enzyme activity. CYP24A1 hydroxylates both 25-hydroxycholecalciferol (25(OH)D3) and 1,25-dihydroxycholecalciferol (1,25(OH)2D3), which is the first step of vitamin D catabolism. An abnormal status of the upregulation of CYP24A1 occurs in many diseases, including chronic kidney disease (CKD). CYP24A1 upregulation in CKD and diminished activation of vitamin D3 contribute to secondary hyperparathyroidism (SHPT), progressive bone deterioration, and soft tissue and cardiovascular calcification. Previous studies have indicated that CYP24A1 inhibition may be an effective strategy to increase endogenous vitamin D activity and decrease SHPT. This study has designed and synthesized a novel C-24 O-methyloxime analogue of vitamin D3 (VD1-6) to have specific CYP24A1 inhibitory properties. VD1-6 did not bind to the vitamin D receptor (VDR) in concentrations up to 10-7 M, assessed by a VDR binding assay. The absence of VDR binding by VD1-6 was confirmed in human embryonic kidney HEK293T cultures through the lack of CYP24A1 induction. However, in silico docking experiments demonstrated that VD1-6 was predicted to have superior binding to CYP24A1, when compared to that of 1,25(OH)2D3. The inhibition of CYP24A1 by VD1-6 was also evident by the synergistic potentiation of 1,25(OH)2D3-mediated transcription and reduced 1,25(OH)2D3 catabolism over 24 h. A further indication of CYP24A1 inhibition by VD1-6 was the reduced accumulation of the 24,25(OH)D3, the first metabolite of 25(OH)D catabolism by CYP24A1. Our findings suggest the potent CYP24A1 inhibitory properties of VD1-6 and its potential for testing as an alternative therapeutic candidate for treating SHPT.
Keywords: CYP24A1; HEK293T; catabolism inhibition; in silico docking; vitamin D3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- St-Arnaud R., Jones G. Vitamin D. Elsevier; Amsterdam, The Netherlands: 2018. CYP24A1: Structure, function, and physiological role; pp. 81–95.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

