Cloning and Characterization of Drosophila melanogaster Juvenile Hormone Epoxide Hydrolases (JHEH) and Their Promoters
- PMID: 35883546
- PMCID: PMC9313241
- DOI: 10.3390/biom12070991
Cloning and Characterization of Drosophila melanogaster Juvenile Hormone Epoxide Hydrolases (JHEH) and Their Promoters
Abstract
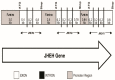
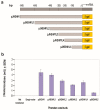
Juvenile hormone epoxide hydrolase (JHEH) plays an important role in the metabolism of JH III in insects. To study the control of JHEH in female Drosophila melanogaster, JHEH 1, 2 and 3 cDNAs were cloned and sequenced. Northern blot analyses showed that the three transcripts are expressed in the head thorax, the gut, the ovaries and the fat body of females. Molecular modeling shows that the enzyme is a homodimer that binds juvenile hormone III acid (JH IIIA) at the catalytic groove better than JH III. Analyses of the three JHEH promoters and expressing short promoter sequences behind a reporter gene (lacZ) in D. melanogaster cell culture identified a JHEH 3 promoter sequence (626 bp) that is 10- and 25-fold more active than the most active promoter sequences of JHEH 2 and JHEH 1, respectively. A transcription factor (TF) Sp1 that is involved in the activation of JHEH 3 promoter sequence was identified. Knocking down Sp1 using dsRNA inhibited the transcriptional activity of this promoter in transfected D. melanogaster cells and JH III and 20HE downregulated the JHEH 3 promoter. On the other hand, JH IIIA and farnesoic acid did not affect the promoter, indicating that JH IIIA is JHEH's preferred substrate. A transgenic D. melanogaster expressing a highly activated JHEH 3 promoter behind a lacZ reporter gene showed promoter transcriptional activity in many D. melanogaster tissues.
Keywords: 3D modeling; RNAi; drosophila; embryo transformation; juvenile hormone epoxide hydrolases promoters; sequencing; tissue culture; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Riddiford L.M. Cellular and molecular actions of juvenile hormone general considerations and premetamorphic actions. Adv. Insect Physiol. 1994;24:213–273.
-
- Wyatt G.R., Davey K.G. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects. Adv. Insect Physiol. 1996;26:1–156.
-
- Stay B., Tobe S.S., Bendena W.G. Allatostatins: Identification, primary structure, function and distribution. Adv. Insect Physiol. 1994;125:267–337.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

