Kallikrein-Related Peptidase 6 (KLK6) as a Contributor toward an Aggressive Cancer Cell Phenotype: A Potential Role in Colon Cancer Peritoneal Metastasis
- PMID: 35883559
- PMCID: PMC9312869
- DOI: 10.3390/biom12071003
Kallikrein-Related Peptidase 6 (KLK6) as a Contributor toward an Aggressive Cancer Cell Phenotype: A Potential Role in Colon Cancer Peritoneal Metastasis
Abstract
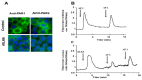
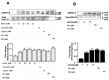
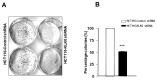
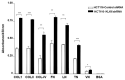
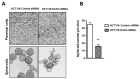
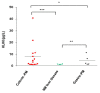
Kallikrein-related peptidases (KLKs) are implicated in many cancer-related processes. KLK6, one of the 15 KLK family members, is a promising biomarker for diagnosis of many cancers and has been associated with poor prognosis of colorectal cancer (CRC) patients. Herein, we evaluated the expression and cellular functions of KLK6 in colon cancer-derived cell lines and in clinical samples from CRC patients. We showed that, although many KLKs transcripts are upregulated in colon cancer-derived cell lines, KLK6, KLK10, and KLK11 are the most highly secreted proteins. KLK6 induced calcium flux in HT29 cells by activation and internalization of protease-activated receptor 2 (PAR2). Furthermore, KLK6 induced extracellular signal-regulated kinases 1 and 2 (ERK1/2) phosphorylation. KLK6 suppression in HCT-116 colon cancer cells decreased the colony formation, increased cell adhesion to extracellular matrix proteins, and reduced spheroid formation and compaction. Immunohistochemistry (IHC) analysis demonstrated ectopic expression of KLK6 in human colon adenocarcinomas but not in normal epithelia. Importantly, high levels of KLK6 protein were detected in the ascites of CRC patients with peritoneal metastasis, but not in benign ascites. These data indicate that KLK6 overexpression is associated with aggressive CRC, and may be applied to differentiate between benign and malignant ascites.
Keywords: colorectal cancer; extracellular matrix; kallikrein-related peptidase 6; metastasis; protease-activated receptors; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Xie Y., Chen L., Lv X., Hou G., Wang Y., Jiang C., Zhu H., Xu N., Wu L., Lou X., et al. The levels of serine proteases in colon tissue interstitial fluid and serum serve as an indicator of colorectal cancer progression. Oncotarget. 2016;7:32592–32606. doi: 10.18632/oncotarget.8693. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

