Preclinical Development of a Therapy for Chronic Traumatic Spinal Cord Injury in Rats Using Human Wharton's Jelly Mesenchymal Stromal Cells: Proof of Concept and Regulatory Compliance
- PMID: 35883596
- PMCID: PMC9319990
- DOI: 10.3390/cells11142153
Preclinical Development of a Therapy for Chronic Traumatic Spinal Cord Injury in Rats Using Human Wharton's Jelly Mesenchymal Stromal Cells: Proof of Concept and Regulatory Compliance
Abstract
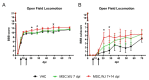
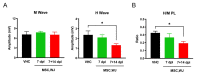
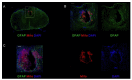
(1) Background: the use of Mesenchymal Stromal Cells (MSC) in emerging therapies for spinal cord injury (SCI) hold the potential to improve functional recovery. However, the development of cell-based medicines is challenging and preclinical studies addressing quality, safety and efficacy must be conducted prior to clinical testing; (2) Methods: herein we present (i) the characterization of the quality attributes of MSC from the Wharton's jelly (WJ) of the umbilical cord, (ii) safety of intrathecal infusion in a 3-month subchronic toxicity assessment study, and (iii) efficacy in a rat SCI model by controlled impaction (100 kdynes) after single (day 7 post-injury) and repeated dose of 1 × 106 MSC,WJ (days 7 and 14 post-injury) with 70-day monitoring by electrophysiological testing, motor function assessment and histology evaluation; (3) Results: no toxicity associated to MSC,WJ infusion was observed. Regarding efficacy, recovery of locomotion was promoted at early time points. Persistence of MSC,WJ was detected early after administration (day 2 post-injection) but not at days 14 and 63 post-injection. (4) Conclusions: the safety profile and signs of efficacy substantiate the suitability of the presented data for inclusion in the Investigational Medicinal Product Dossier for further consideration by the competent Regulatory Authority to proceed with clinical trials.
Keywords: advanced therapy; animal model; cell therapy; cell-based therapy; good laboratory practice; mesenchymal stromal cells; preclinical research; spinal cord injury; stem cells; translational medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

