Upregulated Ca2+ Release from the Endoplasmic Reticulum Leads to Impaired Presynaptic Function in Familial Alzheimer's Disease
- PMID: 35883609
- PMCID: PMC9315668
- DOI: 10.3390/cells11142167
Upregulated Ca2+ Release from the Endoplasmic Reticulum Leads to Impaired Presynaptic Function in Familial Alzheimer's Disease
Abstract
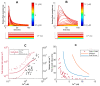
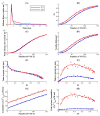
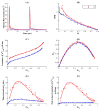
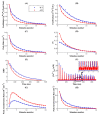
Neurotransmitter release from presynaptic terminals is primarily regulated by rapid Ca2+ influx through membrane-resident voltage-gated Ca2+ channels (VGCCs). Moreover, accumulating evidence indicates that the endoplasmic reticulum (ER) is extensively present in axonal terminals of neurons and plays a modulatory role in synaptic transmission by regulating Ca2+ levels. Familial Alzheimer's disease (FAD) is marked by enhanced Ca2+ release from the ER and downregulation of Ca2+ buffering proteins. However, the precise consequence of impaired Ca2+ signaling within the vicinity of VGCCs (active zone (AZ)) on exocytosis is poorly understood. Here, we perform in silico experiments of intracellular Ca2+ signaling and exocytosis in a detailed biophysical model of hippocampal synapses to investigate the effect of aberrant Ca2+ signaling on neurotransmitter release in FAD. Our model predicts that enhanced Ca2+ release from the ER increases the probability of neurotransmitter release in FAD. Moreover, over very short timescales (30-60 ms), the model exhibits activity-dependent and enhanced short-term plasticity in FAD, indicating neuronal hyperactivity-a hallmark of the disease. Similar to previous observations in AD animal models, our model reveals that during prolonged stimulation (~450 ms), pathological Ca2+ signaling increases depression and desynchronization with stimulus, causing affected synapses to operate unreliably. Overall, our work provides direct evidence in support of a crucial role played by altered Ca2+ homeostasis mediated by intracellular stores in FAD.
Keywords: Alzheimer’s; IP3R; asynchronous release; depression; endoplasmic reticulum; facilitation; neuronal calcium signaling; neurotransmitter release; short-term plasticity; synaptic transmission; synchronous release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Altered neuronal group 1 metabotropic glutamate receptor- and endoplasmic reticulum-mediated Ca2+ signaling in two rodent models of Alzheimer's disease.Neurosci Lett. 2024 Feb 16;823:137664. doi: 10.1016/j.neulet.2024.137664. Epub 2024 Feb 2. Neurosci Lett. 2024. PMID: 38309326
-
Presynaptic endoplasmic reticulum regulates short-term plasticity in hippocampal synapses.Commun Biol. 2021 Feb 23;4(1):241. doi: 10.1038/s42003-021-01761-7. Commun Biol. 2021. PMID: 33623091 Free PMC article.
-
Short-Term Facilitation at a Detonator Synapse Requires the Distinct Contribution of Multiple Types of Voltage-Gated Calcium Channels.J Neurosci. 2017 May 10;37(19):4913-4927. doi: 10.1523/JNEUROSCI.0159-17.2017. Epub 2017 Apr 14. J Neurosci. 2017. PMID: 28411270 Free PMC article.
-
Dysregulation of neuronal calcium homeostasis in Alzheimer's disease - A therapeutic opportunity?Biochem Biophys Res Commun. 2017 Feb 19;483(4):998-1004. doi: 10.1016/j.bbrc.2016.09.053. Epub 2016 Sep 15. Biochem Biophys Res Commun. 2017. PMID: 27641664 Free PMC article. Review.
-
Alterations of the Endoplasmic Reticulum (ER) Calcium Signaling Molecular Components in Alzheimer's Disease.Cells. 2020 Dec 1;9(12):2577. doi: 10.3390/cells9122577. Cells. 2020. PMID: 33271984 Free PMC article. Review.
Cited by
-
Calcium channel signalling at neuronal endoplasmic reticulum-plasma membrane junctions.Biochem Soc Trans. 2024 Aug 28;52(4):1617-1629. doi: 10.1042/BST20230819. Biochem Soc Trans. 2024. PMID: 38934485 Free PMC article. Review.
-
Association between the Composition of Drinking Water and Cognitive Function in the Elderly: A Systematic Review.Int J Environ Res Public Health. 2024 Mar 19;21(3):362. doi: 10.3390/ijerph21030362. Int J Environ Res Public Health. 2024. PMID: 38541362 Free PMC article.
-
Mechanisms of Cadmium Neurotoxicity.Int J Mol Sci. 2023 Nov 21;24(23):16558. doi: 10.3390/ijms242316558. Int J Mol Sci. 2023. PMID: 38068881 Free PMC article. Review.
-
Executive dysfunction is associated with altered hippocampal-prefrontal functional connectivity in 3xTg Alzheimer's model mice.Commun Biol. 2025 Aug 6;8(1):1163. doi: 10.1038/s42003-025-08546-2. Commun Biol. 2025. PMID: 40770414 Free PMC article.
-
Differential behaviors of calcium-induced calcium release in one dimensional dendrite by Nernst-Planck equation, cable model and pure diffusion model.Cogn Neurodyn. 2024 Jun;18(3):1285-1305. doi: 10.1007/s11571-023-09952-0. Epub 2023 Apr 6. Cogn Neurodyn. 2024. PMID: 38826668 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

