The Role of Pannexin-1 Channels in HIV and NeuroHIV Pathogenesis
- PMID: 35883688
- PMCID: PMC9323506
- DOI: 10.3390/cells11142245
The Role of Pannexin-1 Channels in HIV and NeuroHIV Pathogenesis
Abstract
The human immunodeficiency virus-1 (HIV) enters the brain shortly after infection, leading to long-term neurological complications in half of the HIV-infected population, even in the current anti-retroviral therapy (ART) era. Despite decades of research, no biomarkers can objectively measure and, more importantly, predict the onset of HIV-associated neurocognitive disorders. Several biomarkers have been proposed; however, most of them only reflect late events of neuronal damage. Our laboratory recently identified that ATP and PGE2, inflammatory molecules released through Pannexin-1 channels, are elevated in the serum of HIV-infected individuals compared to uninfected individuals and other inflammatory diseases. More importantly, high circulating ATP levels, but not PGE2, can predict a decline in cognition, suggesting that HIV-infected individuals have impaired ATP metabolism and associated signaling. We identified that Pannexin-1 channel opening contributes to the high serological ATP levels, and ATP in the circulation could be used as a biomarker of HIV-associated cognitive impairment. In addition, we believe that ATP is a major contributor to chronic inflammation in the HIV-infected population, even in the anti-retroviral era. Here, we discuss the mechanisms associated with Pannexin-1 channel opening within the circulation, as well as within the resident viral reservoirs, ATP dysregulation, and cognitive disease observed in the HIV-infected population.
Keywords: biomarker; comorbidities; cure; dementia; purinergic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
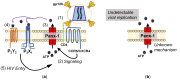
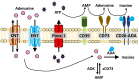
Similar articles
-
Circulating levels of ATP is a biomarker of HIV cognitive impairment.EBioMedicine. 2020 Jan;51:102503. doi: 10.1016/j.ebiom.2019.10.029. Epub 2019 Dec 3. EBioMedicine. 2020. PMID: 31806564 Free PMC article.
-
The role of Pannexin-1 channels and extracellular ATP in the pathogenesis of the human immunodeficiency virus.Purinergic Signal. 2021 Dec;17(4):563-576. doi: 10.1007/s11302-021-09817-3. Epub 2021 Sep 20. Purinergic Signal. 2021. PMID: 34542793 Free PMC article. Review.
-
Bidirectional Associations among Nicotine and Tobacco Smoke, NeuroHIV, and Antiretroviral Therapy.J Neuroimmune Pharmacol. 2020 Dec;15(4):694-714. doi: 10.1007/s11481-019-09897-4. Epub 2019 Dec 13. J Neuroimmune Pharmacol. 2020. PMID: 31834620 Free PMC article. Review.
-
HIV gp120 Protein Increases the Function of Connexin 43 Hemichannels and Pannexin-1 Channels in Astrocytes: Repercussions on Astroglial Function.Int J Mol Sci. 2020 Apr 3;21(7):2503. doi: 10.3390/ijms21072503. Int J Mol Sci. 2020. PMID: 32260308 Free PMC article.
-
Mechanisms of HIV Neuropathogenesis: Role of Cellular Communication Systems.Curr HIV Res. 2016;14(5):400-411. doi: 10.2174/1570162x14666160324124558. Curr HIV Res. 2016. PMID: 27009098 Free PMC article. Review.
Cited by
-
The Double-Edged Effect of Connexins and Pannexins of Glial Cells in Central and Peripheral Nervous System After Nerve Injury.Mol Neurobiol. 2025 May 1. doi: 10.1007/s12035-025-04991-6. Online ahead of print. Mol Neurobiol. 2025. PMID: 40310549 Review.
-
Chronic brain damage in HIV-infected individuals under antiretroviral therapy is associated with viral reservoirs, sulfatide release, and compromised cell-to-cell communication.Cell Mol Life Sci. 2023 Apr 4;80(4):116. doi: 10.1007/s00018-023-04757-0. Cell Mol Life Sci. 2023. PMID: 37016051 Free PMC article.
-
Influence of the Gut Microbiota on the Development of Neurodegenerative Diseases.Mediators Inflamm. 2022 Sep 30;2022:3300903. doi: 10.1155/2022/3300903. eCollection 2022. Mediators Inflamm. 2022. PMID: 36248189 Free PMC article. Review.
-
Mechanisms of HIV-mediated blood-brain barrier compromise and leukocyte transmigration under the current antiretroviral era.iScience. 2024 Feb 15;27(3):109236. doi: 10.1016/j.isci.2024.109236. eCollection 2024 Mar 15. iScience. 2024. PMID: 38487019 Free PMC article.
References
-
- Barré-Sinoussi F., Chermann J.C., Rey F., Nugeyre M.T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C., et al. Isolation of a T-Lymphotropic Retrovirus from a Patient at Risk for Acquired Immune Deficiency Syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. - DOI - PubMed
-
- Finzi D., Blankson J.N., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T.C., Smith K., Lisziewicz J., Lori F., Flexner C., et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. - DOI - PubMed
-
- Valcour V., Chalermchai T., Sailasuta N., Marovich M., Lerdlum S., Suttichom D., Suwanwela N.C., Jagodzinski L.L., Michael N.L., Spudich S., et al. Central Nervous System Viral Invasion and Inflammation During Acute HIV Infection. J. Infect. Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. - DOI - PMC - PubMed
-
- Davey R.T., Bhat N., Yoder C., Chun T.-W., Metcalf J.A., Dewar R., Natarajan V., Lempicki R.A., Adelsberger J.W., Miller K.D., et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. - DOI - PMC - PubMed
-
- Joos B., Fischer M., Kuster H., Pillai S.K., Wong J.K., Böni J., Hirschel B., Weber R., Trkola A., Günthard H.F., et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc. Natl. Acad. Sci. USA. 2008;105:16725–16730. doi: 10.1073/pnas.0804192105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

