Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas aeruginosa LPS-Activated THP-1 Macrophages
- PMID: 35883820
- PMCID: PMC9311800
- DOI: 10.3390/antiox11071330
Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas aeruginosa LPS-Activated THP-1 Macrophages
Abstract
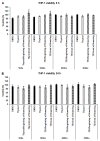
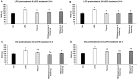
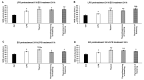
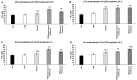
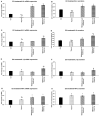
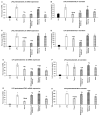
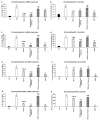
Thyme (Thymus vulgaris L.) essential oil (TEO) is widely used as an alternative therapy especially for infections of the upper respiratory tract. TEO possesses antiviral, antibacterial, and antifungal properties. The emerging antibiotic resistance of bacterial strains, including Pseudomonas aeruginosa, has prompted the urge to find alternative treatments. In the present study, we examined the anti-inflammatory and antioxidant effects of thymol, the main compound of TEO, and two TEOs prepared at the beginning and at the end of the flowering period that may make these oils promising candidates as complementary or alternative therapies against P. aeruginosa infections. The activity measurements of the antioxidant enzymes peroxidase (PX), catalase (CAT), and superoxide dismutase (SOD) as well as the determination of total antioxidant capacity of P. aeruginosa-activated THP-1 cells revealed that thymol and both TEOs increased CAT and SOD activity as well as the antioxidant capacity of the THP-1 cells. The measurements of the proinflammatory cytokine mRNA expression and secreted protein level of LPS-activated THP-1 cells showed that from the two TEOs, only TEO prepared at the beginning of the flowering period acted as a potent inhibitor of the synthesis of IL-6, IL-8, IL-β, and TNF-α. Our results suggest that not only thymol, but also the synergism or the antagonistic effects of the additional compounds of the essential oils are responsible for the anti-inflammatory activity of TEOs.
Keywords: antioxidant capacity; cytokines; essential oil; inflammation; macrophage; thyme.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Tariq S., Wani S., Rasool W., Shafi K., Bhat M.A., Prabhakar A., Shalla A.H., Rather M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019;134:103580. doi: 10.1016/j.micpath.2019.103580. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

