Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease
- PMID: 35883847
- PMCID: PMC9311633
- DOI: 10.3390/antiox11071356
Tubular Mitochondrial Dysfunction, Oxidative Stress, and Progression of Chronic Kidney Disease
Abstract
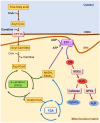
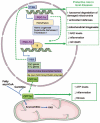
Acute kidney injury (AKI) and chronic kidney disease (CKD) are interconnected conditions, and CKD is projected to become the fifth leading global cause of death by 2040. New therapeutic approaches are needed. Mitochondrial dysfunction and oxidative stress have emerged as drivers of kidney injury in acute and chronic settings, promoting the AKI-to-CKD transition. In this work, we review the role of mitochondrial dysfunction and oxidative stress in AKI and CKD progression and discuss novel therapeutic approaches. Specifically, evidence for mitochondrial dysfunction in diverse models of AKI (nephrotoxicity, cytokine storm, and ischemia-reperfusion injury) and CKD (diabetic kidney disease, glomerulopathies) is discussed; the clinical implications of novel information on the key role of mitochondria-related transcriptional regulators peroxisome proliferator-activated receptor gamma coactivator 1-alpha, transcription factor EB (PGC-1α, TFEB), and carnitine palmitoyl-transferase 1A (CPT1A) in kidney disease are addressed; the current status of the clinical development of therapeutic approaches targeting mitochondria are updated; and barriers to the clinical development of mitochondria-targeted interventions are discussed, including the lack of clinical diagnostic tests that allow us to categorize the baseline renal mitochondrial dysfunction/mitochondrial oxidative stress and to monitor its response to therapeutic intervention. Finally, key milestones for further research are proposed.
Keywords: PGC-1α; acute kidney injury; chronic kidney disease; mitochondria; oxidative stress.
Conflict of interest statement
A.O. has received grants from Sanofi and consultancy or speaker fees or travel support from Advicciene, Astellas, Astrazeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, and Vifor Fresenius Medical Care Renal Pharma and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes. The rest of the authors have nothing to disclose.
Figures
References
-
- Ortiz A., Asociación Información Enfermedades Renales Genéticas (AIRG-E) European Kidney Patients’ Federation. Federación Nacional de Asociaciones para la Lucha Contra las Enfermedades del Riñón (ALCER) Fundación Renal Íñigo Álvarez de Toledo (FRIAT) Red de Investigación Renal (REDINREN) Resultados en Salud 2040 (RICORS2040) Sociedad Española de Nefrología (SENEFRO) Council, Sociedad Española de Trasplante (SET) Council. Organización Nacional de Trasplantes (ONT) RICORS2040: The need for collaborative research in chronic kidney disease. Clin. Kidney J. 2022;15:372–387. doi: 10.1093/ckj/sfab170. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

