How Aging and Oxidative Stress Influence the Cytopathic and Inflammatory Effects of SARS-CoV-2 Infection: The Role of Cellular Glutathione and Cysteine Metabolism
- PMID: 35883857
- PMCID: PMC9311797
- DOI: 10.3390/antiox11071366
How Aging and Oxidative Stress Influence the Cytopathic and Inflammatory Effects of SARS-CoV-2 Infection: The Role of Cellular Glutathione and Cysteine Metabolism
Abstract
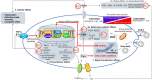
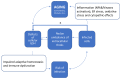
SARS-CoV-2 infection can cause a severe respiratory distress syndrome with inflammatory and thrombotic complications, the severity of which increases with patients' age and presence of comorbidity. The reasons for an age-dependent increase in the risk of severe COVID-19 could be many. These include defects in the homeostatic processes that control the cellular redox and its pivotal role in sustaining the immuno-inflammatory response to the host and the protection against oxidative stress and tissue degeneration. Pathogens may take advantage of such age-dependent abnormalities. Alterations of the thiol redox balance in the lung tissue and lining fluids may influence the risk of infection, and the host capability to respond to pathogens and to avoid severe complications. SARS-CoV-2, likewise other viruses, such as HIV, influenza, and HSV, benefits in its replication cycle of pro-oxidant conditions that the same viral infection seems to induce in the host cell with mechanisms that remain poorly understood. We recently demonstrated that the pro-oxidant effects of SARS-CoV-2 infection are associated with changes in the cellular metabolism and transmembrane fluxes of Cys and GSH. These appear to be the consequence of an increased use of Cys in viral protein synthesis and to ER stress pathway activation that interfere with transcription factors, as Nrf2 and NFkB, important to coordinate the metabolism of GSH with other aspects of the stress response and with the pro-inflammatory effects of this virus in the host cell. This narrative review article describes these cellular and molecular aspects of SARS-CoV-2 infection, and the role that antivirals and cytoprotective agents such as N-acetyl cysteine may have to limit the cytopathic effects of this virus and to recover tissue homeostasis after infection.
Keywords: SARS-CoV-2; aging; cellular redox; cytokines; glutathione; inflammation; lung diseases; oxidative stress; thiols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

