An In-Depth Study on the Metabolite Profile and Biological Properties of Primula auriculata Extracts: A Fascinating Sparkle on the Way from Nature to Functional Applications
- PMID: 35883868
- PMCID: PMC9312287
- DOI: 10.3390/antiox11071377
An In-Depth Study on the Metabolite Profile and Biological Properties of Primula auriculata Extracts: A Fascinating Sparkle on the Way from Nature to Functional Applications
Abstract
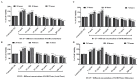
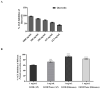
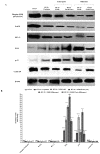
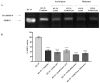
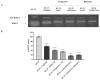
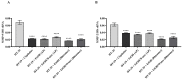
The biological activity of the aerial part and rhizomes of Primula auriculata were assessed for the first time. The biological activities (antioxidant properties, enzyme inhibition, and AGE inhibition) as well as the phenolic and flavonoid contents of the ethyl acetate, ethanol, hydro-ethanol and water extracts of P. auriculata aerial parts and rhizomes were determined. Cell viability assays and gelatin zymography were also performed for MMP-2/-9 to determine the molecular mechanisms of action. The gene expression for MMPs was described with RT-PCR. The levels of various proteins, including phospho-Nf-κB, BCL-2, BAX, p-53, and cyclin D1 as well as RAGE were measured using Western blot analysis. The hydro-ethanol extract of the aerial part possessed the highest phenolic (56.81 mg GAE/g) and flavonoid (63.92 mg RE/g) contents. In-depth profiling of the specialized metabolites by ultra-high-performance liquid chromatography-high-resolution mass spectrometry (UHPLC-HRMS) allowed for the identification and annotation of 65 compounds, including phenolic acids and glycosides, flavones, flavonols, chalcones, dihydrochalcones, and saponins. The hydro-ethanol extract of the aerial parts (132.65, 180.87, 172.46, and 108.37 mg TE/g, for the DPPH, ABTS, CUPRAC, and FRAP assays, respectively) and the ethanol extract of the rhizomes (415.06, 638.30, 477.77, and 301.02 mg TE/g, for the DPPH, ABTS, CUPRAC, and FRAP assays, respectively) exhibited the highest free radical scavenging and reducing activities. The ethanol and hydro-ethanol extracts of both the P. auriculata aerial part and rhizomes exhibited higher inhibitory activity against acetylcholinesterase, while the hydro-ethanol extracts (1.16 mmol ACAE/g, for both the aerial part and rhizomes extracts) were more active in the inhibition of α-glucosidase. After the treatment of an HT-29 colorectal cancer cell line with the extracts, the apoptosis mechanism was initiated, the integrity of the ECM was remodeled, and cell proliferation was also taken under control. In this way, Primula extracts were shown to be potential drug sources in the treatment of colorectal cancer. They were also detected as natural MMP inhibitors. The findings presented in the present study appraise the bioactivity of P. auriculata, an understudied species. Additional assessment is required to evaluate the cytotoxicity of P. auriculata as well as its activity in ex vivo systems.
Keywords: AGE inhibition; Primula; anti-apoptotic pathway; antioxidant; glucosidase; phenolics; phospho-NF-KB.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nirmala C., Shahar B., Dolma N., Santosh O. Promising underutilized wild plants of cold desert Ladakh, India for nutritional security and health benefits. Appl. Food Res. 2022;2:100145. doi: 10.1016/j.afres.2022.100145. - DOI
-
- Singh P.A., Bajwa N., Chinnam S., Chandan A., Baldi A. An overview of some important deliberations to promote medicinal plants cultivation. J. Appl. Res. Med. Aromat. Plants. 2022;31:100400. doi: 10.1016/j.jarmap.2022.100400. - DOI
-
- Mohammed A., Tajuddeen N. Antidiabetic compounds from medicinal plants traditionally used for the treatment of diabetes in Africa: A review update (2015–2020) S. Afr. J. Bot. 2022;146:585–602. doi: 10.1016/j.sajb.2021.11.018. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

