Focus on Nitric Oxide Homeostasis: Direct and Indirect Enzymatic Regulation of Protein Denitrosation Reactions in Plants
- PMID: 35883902
- PMCID: PMC9311986
- DOI: 10.3390/antiox11071411
Focus on Nitric Oxide Homeostasis: Direct and Indirect Enzymatic Regulation of Protein Denitrosation Reactions in Plants
Abstract
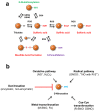
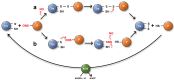
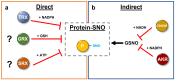
Protein cysteines (Cys) undergo a multitude of different reactive oxygen species (ROS), reactive sulfur species (RSS), and/or reactive nitrogen species (RNS)-derived modifications. S-nitrosation (also referred to as nitrosylation), the addition of a nitric oxide (NO) group to reactive Cys thiols, can alter protein stability and activity and can result in changes of protein subcellular localization. Although it is clear that this nitrosative posttranslational modification (PTM) regulates multiple signal transduction pathways in plants, the enzymatic systems that catalyze the reverse S-denitrosation reaction are poorly understood. This review provides an overview of the biochemistry and regulation of nitro-oxidative modifications of protein Cys residues with a focus on NO production and S-nitrosation. In addition, the importance and recent advances in defining enzymatic systems proposed to be involved in regulating S-denitrosation are addressed, specifically cytosolic thioredoxins (TRX) and the newly identified aldo-keto reductases (AKR).
Keywords: Arabidopsis thaliana; S-Nitrosoglutathione reductase (GSNOR); S-nitrosation; aldo-keto reductases (AKRs); glutaredoxins (GRXs); posttranslational modifications; reactive nitrogen species; thioredoxins (TRXs).
Conflict of interest statement
The authors declare no conflict of interest
Figures




Similar articles
-
Thioredoxins: Emerging Players in the Regulation of Protein S-Nitrosation in Plants.Plants (Basel). 2020 Oct 24;9(11):1426. doi: 10.3390/plants9111426. Plants (Basel). 2020. PMID: 33114295 Free PMC article. Review.
-
Quantitative Proteome Profiling of a S-Nitrosoglutathione Reductase (GSNOR) Null Mutant Reveals a New Class of Enzymes Involved in Nitric Oxide Homeostasis in Plants.Front Plant Sci. 2021 Dec 7;12:787435. doi: 10.3389/fpls.2021.787435. eCollection 2021. Front Plant Sci. 2021. PMID: 34956283 Free PMC article.
-
ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications.Free Radic Res. 2018 May;52(5):507-543. doi: 10.1080/10715762.2018.1457217. Epub 2018 Apr 19. Free Radic Res. 2018. PMID: 29589770 Review.
-
S-Nitrosoglutathione Reductase-The Master Regulator of Protein S-Nitrosation in Plant NO Signaling.Plants (Basel). 2019 Feb 21;8(2):48. doi: 10.3390/plants8020048. Plants (Basel). 2019. PMID: 30795534 Free PMC article. Review.
-
Measurement of S-Nitrosoglutathione Reductase Activity in Plants.Methods Mol Biol. 2020;2057:45-59. doi: 10.1007/978-1-4939-9790-9_5. Methods Mol Biol. 2020. PMID: 31595469
Cited by
-
Nitric oxide (NO) modulates low temperature-stress signaling via S-nitrosation, a NO PTM, inducing ethylene biosynthesis inhibition leading to enhanced post-harvest shelf-life of agricultural produce.Physiol Mol Biol Plants. 2023 Dec;29(12):2051-2065. doi: 10.1007/s12298-023-01371-z. Epub 2023 Nov 2. Physiol Mol Biol Plants. 2023. PMID: 38222283 Free PMC article. Review.
-
Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection.Int J Mol Sci. 2025 Feb 27;26(5):2087. doi: 10.3390/ijms26052087. Int J Mol Sci. 2025. PMID: 40076711 Free PMC article. Review.
-
Sodium nitroprusside modulates oxidative and nitrosative processes in Lycopersicum esculentum L. under drought stress.Plant Cell Rep. 2024 May 28;43(6):152. doi: 10.1007/s00299-024-03238-3. Plant Cell Rep. 2024. PMID: 38806834 Free PMC article.
-
Reactive Byproducts of Plant Redox Metabolism and Protein Functions.Acta Naturae. 2024 Oct-Dec;16(4):48-61. doi: 10.32607/actanaturae.27477. Acta Naturae. 2024. PMID: 39877007 Free PMC article.
-
Nitric Oxide in Plant Functioning: Metabolism, Signaling, and Responses to Infestation with Ecdysozoa Parasites.Biology (Basel). 2023 Jun 28;12(7):927. doi: 10.3390/biology12070927. Biology (Basel). 2023. PMID: 37508359 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

