Adenomesenteritis following SARS-CoV-2 Vaccination in Children: A Case Report and Review of The Literature
- PMID: 35883977
- PMCID: PMC9321070
- DOI: 10.3390/children9070993
Adenomesenteritis following SARS-CoV-2 Vaccination in Children: A Case Report and Review of The Literature
Abstract
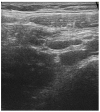
At present, the vaccine authorized in children aged 5 years and older is the BNT162b2 messenger RNA COVID-19 vaccine. Unlike adults, there is limited data available in the pediatric age describing adverse events after vaccine. We report a case of adenomesenteritis in a young girl following the first dose of vaccine.
Keywords: SARS-CoV-2; adverse event; children; lymphadenopathy; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Reduced humoral response 3 months following BNT162b2 vaccination in SARS-CoV-2 uninfected residents of long-term care facilities.Age Ageing. 2022 May 1;51(5):afac101. doi: 10.1093/ageing/afac101. Age Ageing. 2022. PMID: 35595256 Free PMC article.
-
Safety Monitoring after the BNT162b2 COVID-19 Vaccine among Adults Aged 75 Years or Older.J Korean Med Sci. 2021 Nov 22;36(45):e318. doi: 10.3346/jkms.2021.36.e318. J Korean Med Sci. 2021. PMID: 34811980 Free PMC article.
-
Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study.Lancet Haematol. 2021 Aug;8(8):e583-e592. doi: 10.1016/S2352-3026(21)00169-1. Epub 2021 Jul 2. Lancet Haematol. 2021. PMID: 34224668 Free PMC article.
-
Development of Graves' Disease After SARS-CoV-2 mRNA Vaccination: A Case Report and Literature Review.Front Public Health. 2021 Nov 23;9:778964. doi: 10.3389/fpubh.2021.778964. eCollection 2021. Front Public Health. 2021. PMID: 34888290 Free PMC article. Review.
-
New-onset pediatric nephrotic syndrome following Pfizer-BioNTech SARS-CoV-2 vaccination: a case report and literature review.CEN Case Rep. 2022 May;11(2):242-246. doi: 10.1007/s13730-021-00656-0. Epub 2021 Nov 15. CEN Case Rep. 2022. PMID: 34782983 Free PMC article. Review.
References
-
- Seow J., Graham C., Merrick B., Acors S., Pickering S., Kja S., Hemmings O., O’Byrne A., Kouphou N., Galao R.P., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2021;5:159. doi: 10.1038/s41564-020-00813-8. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

