Acetylome and Succinylome Profiling of Edwardsiella tarda Reveals Key Roles of Both Lysine Acylations in Bacterial Antibiotic Resistance
- PMID: 35884095
- PMCID: PMC9312108
- DOI: 10.3390/antibiotics11070841
Acetylome and Succinylome Profiling of Edwardsiella tarda Reveals Key Roles of Both Lysine Acylations in Bacterial Antibiotic Resistance
Abstract
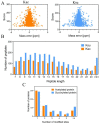
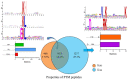
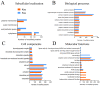
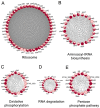
The antibiotic resistance of Edwardsiella tarda is becoming increasingly prevalent, and thus novel antimicrobial strategies are being sought. Lysine acylation has been demonstrated to play an important role in bacterial physiological functions, while its role in bacterial antibiotic resistance remains largely unclear. In this study, we investigated the lysine acetylation and succinylation profiles of E. tarda strain EIB202 using affinity antibody purification combined with LC-MS/MS. A total of 1511 lysine-acetylation sites were identified on 589 proteins, and 2346 lysine-succinylation sites were further identified on 692 proteins of this pathogen. Further bioinformatic analysis showed that both post-translational modifications (PTMs) were enriched in the tricarboxylic acid (TCA) cycle, pyruvate metabolism, biosynthesis, and carbon metabolism. In addition, 948 peptides of 437 proteins had overlapping associations with multiple metabolic pathways. Moreover, both acetylation and succinylation were found in many antimicrobial resistance (AMR) proteins, suggesting their potentially vital roles in antibiotic resistance. In general, our work provides insights into the acetylome and succinylome features responsible for the antibiotic resistance mechanism of E. tarda, and the results may facilitate future investigations into the pathogenesis of this bacterium.
Keywords: Edwardsiella tarda; acetylome; antibiotic resistance; succinylome.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Comprehensive analysis of the lysine acetylome in Aeromonas hydrophila reveals cross-talk between lysine acetylation and succinylation in LuxS.Emerg Microbes Infect. 2019;8(1):1229-1239. doi: 10.1080/22221751.2019.1656549. Emerg Microbes Infect. 2019. PMID: 31448697 Free PMC article.
-
Identification of Lysine Succinylome and Acetylome in the Vancomycin-Intermediate Staphylococcus aureus XN108.Microbiol Spectr. 2022 Dec 21;10(6):e0348122. doi: 10.1128/spectrum.03481-22. Epub 2022 Nov 14. Microbiol Spectr. 2022. PMID: 36374118 Free PMC article.
-
First Succinylome Profiling of Vibrio alginolyticus Reveals Key Role of Lysine Succinylation in Cellular Metabolism and Virulence.Front Cell Infect Microbiol. 2021 Feb 5;10:626574. doi: 10.3389/fcimb.2020.626574. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33614530 Free PMC article.
-
Integrated Succinylome and Metabolome Profiling Reveals Crucial Role of S-Ribosylhomocysteine Lyase in Quorum Sensing and Metabolism of Aeromonas hydrophila.Mol Cell Proteomics. 2019 Feb;18(2):200-215. doi: 10.1074/mcp.RA118.001035. Epub 2018 Oct 23. Mol Cell Proteomics. 2019. PMID: 30352804 Free PMC article.
-
Changes in the Acetylome and Succinylome of Bacillus subtilis in Response to Carbon Source.PLoS One. 2015 Jun 22;10(6):e0131169. doi: 10.1371/journal.pone.0131169. eCollection 2015. PLoS One. 2015. PMID: 26098117 Free PMC article.
Cited by
-
Acetyl-proteome profiling revealed the role of lysine acetylation in erythromycin resistance of Staphylococcus aureus.Heliyon. 2024 Jul 26;10(15):e35326. doi: 10.1016/j.heliyon.2024.e35326. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170456 Free PMC article.
-
Lysine acetylation regulates the AT-rich DNA possession ability of H-NS.Nucleic Acids Res. 2024 Feb 28;52(4):1645-1660. doi: 10.1093/nar/gkad1172. Nucleic Acids Res. 2024. PMID: 38059366 Free PMC article.
-
Recent Contributions of Proteomics to Our Understanding of Reversible Nε-Lysine Acylation in Bacteria.J Proteome Res. 2024 Aug 2;23(8):2733-2749. doi: 10.1021/acs.jproteome.3c00912. Epub 2024 Mar 5. J Proteome Res. 2024. PMID: 38442041 Free PMC article. Review.
-
A Systematic Study of Lysine Succinylation in the Pathogenic Bacterium Vibrio harveyi in Aquatic Animals.Molecules. 2025 May 31;30(11):2418. doi: 10.3390/molecules30112418. Molecules. 2025. PMID: 40509307 Free PMC article.
-
Lactiplantibacillus plantarum Ameliorated Growth Performance and Intestinal Inflammation by Modulating Gut Microbial Structure and Function of Yellow River Carp (Cyprinus carpio).Probiotics Antimicrob Proteins. 2025 May 30. doi: 10.1007/s12602-025-10589-0. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40445295
References
-
- Zhang X., Ning Z., Mayne J., Yang Y., Deeke S.A., Walker K., Farnsworth C.L., Stokes M.P., Couture J.F., Mack D., et al. Widespread protein lysine acetylation in gut microbiome and its alterations in patients with Crohn’s disease. Nat. Commun. 2020;11:4120. doi: 10.1038/s41467-020-17916-9. - DOI - PMC - PubMed
-
- Castano-Cerezo S., Bernal V., Post H., Fuhrer T., Cappadona S., Sanchez-Diaz N.C., Sauer U., Heck A.J., Altelaar A.F., Canovas M. Protein acetylation affects acetate metabolism, motility and acid stress response in Escherichia coli. Mol. Syst. Biol. 2014;10:762. doi: 10.15252/msb.20145227. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

