Novel 1,2,3-Triazole-sulphadiazine-ZnO Hybrids as Potent Antimicrobial Agents against Carbapenem Resistant Bacteria
- PMID: 35884170
- PMCID: PMC9312158
- DOI: 10.3390/antibiotics11070916
Novel 1,2,3-Triazole-sulphadiazine-ZnO Hybrids as Potent Antimicrobial Agents against Carbapenem Resistant Bacteria
Abstract
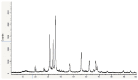
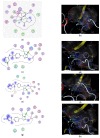
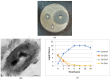
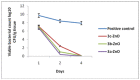
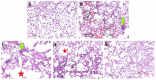
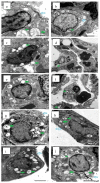
Bacterial pneumonia is considered one of the most virulent diseases with high morbidity and mortality rates, especially in hospitalized patients. Moreover, bacterial resistance increased over the last decades which limited the therapy options to carbapenem antibiotics. Hence, the metallo-β-lactamase-producing bacteria were deliberated as the most deadly and ferocious infectious agents. Sulphadiazine-ZnO hybrids biological activity was explored in vitro and in vivo against metallo-β-lactamases (MBLs) producing Klebsiella pneumoniae. Docking studies against NDM-1 and IMP-1 MBLs revealed the superior activity of the 3a compound in inhibiting both MBLs enzymes in a valid reliable docking approach. The MBLs inhibition enzyme assay revealed the remarkable sulphadiazine-ZnO hybrids inhibitory effect against NDM-1 and IMP-1 MBLs. The tested compounds inhibited the enzymes both competitively and noncompetitively. Compound 3b-ZnO showed the highest antibacterial activity against the tested metallo-β-lactamase producers with an inhibition zone (IZ) diameter reaching 43 mm and a minimum inhibitory concentration (MIC) reaching 2 µg/mL. Sulphadiazine-ZnO hybrids were tested for their in vitro cytotoxicity in a normal lung cell line (BEAS-2Bs cell line). Higher cell viability was observed with 3b-ZnO. Biodistribution of the sulphadiazine-ZnO hybrids in the lungs of uninfected rats revealed that both [124I]3a-ZnO and [124I]3b-ZnO hybrids remained detectable within the rats' lungs after 24 h of endotracheal aerosolization. Moreover, the residence duration in the lungs of [124I]3b-ZnO (t1/2 4.91 h) was 85.3%. The histopathological investigations confirmed that compound 3b-ZnO has significant activity in controlling bacterial pneumonia infection in rats.
Keywords: IMP; NDM; docking; enzyme assay; in vivo; pneumonia; sulphadiazine-ZnO hybrids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Wiegand S.B., Traeger L., Nguyen H.K., Rouillard K.R., Fischbach A., Zadek F., Ichinose F., Schoenfisch M.H., Carroll R.W., Blochad D.B., et al. Antimicrobial effects of nitric oxide in murine models of Klebsiella pneumonia. Redox Biol. 2021;39:101826. doi: 10.1016/j.redox.2020.101826. - DOI - PMC - PubMed
-
- Glover R.E., Petticrew M.P., Mays N.B., Thompson C. How pharmaceutical and diagnostic stakeholders construct policy solutions to a public health ‘crisis’: An analysis of submissions to a United Kingdom House of Commons inquiry into antimicrobial resistance. Crit. Public Health. 2022:1–10. doi: 10.1080/09581596.2022.2026296. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

