The Association between Biofilm Formation and Antimicrobial Resistance with Possible Ingenious Bio-Remedial Approaches
- PMID: 35884186
- PMCID: PMC9312340
- DOI: 10.3390/antibiotics11070930
The Association between Biofilm Formation and Antimicrobial Resistance with Possible Ingenious Bio-Remedial Approaches
Abstract
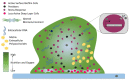
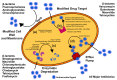
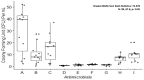
Biofilm has garnered a lot of interest due to concerns in various sectors such as public health, medicine, and the pharmaceutical industry. Biofilm-producing bacteria show a remarkable drug resistance capability, leading to an increase in morbidity and mortality. This results in enormous economic pressure on the healthcare sector. The development of biofilms is a complex phenomenon governed by multiple factors. Several attempts have been made to unravel the events of biofilm formation; and, such efforts have provided insights into the mechanisms to target for the therapy. Owing to the fact that the biofilm-state makes the bacterial pathogens significantly resistant to antibiotics, targeting pathogens within biofilm is indeed a lucrative prospect. The available drugs can be repurposed to eradicate the pathogen, and as a result, ease the antimicrobial treatment burden. Biofilm formers and their infections have also been found in plants, livestock, and humans. The advent of novel strategies such as bioinformatics tools in treating, as well as preventing, biofilm formation has gained a great deal of attention. Development of newfangled anti-biofilm agents, such as silver nanoparticles, may be accomplished through omics approaches such as transcriptomics, metabolomics, and proteomics. Nanoparticles' anti-biofilm properties could help to reduce antimicrobial resistance (AMR). This approach may also be integrated for a better understanding of biofilm biology, guided by mechanistic understanding, virtual screening, and machine learning in silico techniques for discovering small molecules in order to inhibit key biofilm regulators. This stimulated research is a rapidly growing field for applicable control measures to prevent biofilm formation. Therefore, the current article discusses the current understanding of biofilm formation, antibiotic resistance mechanisms in bacterial biofilm, and the novel therapeutic strategies to combat biofilm-mediated infections.
Keywords: AMR; biofilm; biofilm control; extracellular polymeric substances; multidrug resistance; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Burnett-Boothroyd S.C., McCarthy B.J. 13-Antimicrobial treatments of textiles for hygiene and infection control applications: An Industrial perspective. In: McCarthy B.J., editor. Textiles for Hygiene and Infection Control. Woodhead Publishing; Sawston, UK: 2011. pp. 196–209. (Woodhead Publishing Series in Textiles).
-
- Saga T., Yamaguchi K. History of antimicrobial agents and resistant. Jpn. Med. Assoc. J. 2009;52:103–108.
-
- Ellis A. Overcoming Resistance: A Rational Emotive Behavior Therapy Integrated Approach. 2nd ed. Springer Publishing Company; Cham, Switzerland: 2002.
-
- Percival S.L., Malic S., Cruz H., Williams D.W. Introduction to biofilms. In: Percival S., Knottenbelt D., Cochrane C., editors. Biofilms and Veterinary Medicine. Springer; Berlin/Heidelberg, Germany: 2011. pp. 41–68. (Springer Series on Biofilms).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

