Utilizing Electrochemical-Based Sensing Approaches for the Detection of SARS-CoV-2 in Clinical Samples: A Review
- PMID: 35884276
- PMCID: PMC9312918
- DOI: 10.3390/bios12070473
Utilizing Electrochemical-Based Sensing Approaches for the Detection of SARS-CoV-2 in Clinical Samples: A Review
Abstract
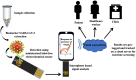
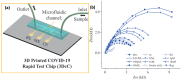
The development of precise and efficient diagnostic tools enables early treatment and proper isolation of infected individuals, hence limiting the spread of coronavirus disease 2019 (COVID-19). The standard diagnostic tests used by healthcare workers to diagnose severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection have some limitations, including longer detection time, the need for qualified individuals, and the use of sophisticated bench-top equipment, which limit their use for rapid SARS-CoV-2 assessment. Advances in sensor technology have renewed the interest in electrochemical biosensors miniaturization, which provide improved diagnostic qualities such as rapid response, simplicity of operation, portability, and readiness for on-site screening of infection. This review gives a condensed overview of the current electrochemical sensing platform strategies for SARS-CoV-2 detection in clinical samples. The fundamentals of fabricating electrochemical biosensors, such as the chosen electrode materials, electrochemical transducing techniques, and sensitive biorecognition molecules, are thoroughly discussed in this paper. Furthermore, we summarised electrochemical biosensors detection strategies and their analytical performance on diverse clinical samples, including saliva, blood, and nasopharyngeal swab. Finally, we address the employment of miniaturized electrochemical biosensors integrated with microfluidic technology in viral electrochemical biosensors, emphasizing its potential for on-site diagnostics applications.
Keywords: COVID-19; SARS-CoV-2; diagnostic methods; electrochemical biosensor; microfluidic electrochemical devices; miniaturised electrochemical sensor; point of care (POC).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Lab-on-a-Disc for Point-of-Care Infection Diagnostics.Acc Chem Res. 2021 Oct 5;54(19):3643-3655. doi: 10.1021/acs.accounts.1c00367. Epub 2021 Sep 13. Acc Chem Res. 2021. PMID: 34516092
-
Voltammetric-based immunosensor for the detection of SARS-CoV-2 nucleocapsid antigen.Mikrochim Acta. 2021 May 26;188(6):199. doi: 10.1007/s00604-021-04867-1. Mikrochim Acta. 2021. PMID: 34041585 Free PMC article.
-
Electrochemical biosensors for SARS-CoV-2 detection: Voltametric or impedimetric transduction?Bioelectrochemistry. 2022 Oct;147:108190. doi: 10.1016/j.bioelechem.2022.108190. Epub 2022 Jun 11. Bioelectrochemistry. 2022. PMID: 35738049 Free PMC article. Review.
-
Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva.Biosens Bioelectron. 2021 Jan 1;171:112686. doi: 10.1016/j.bios.2020.112686. Epub 2020 Oct 3. Biosens Bioelectron. 2021. PMID: 33086175 Free PMC article.
-
Low-Cost Biosensor Technologies for Rapid Detection of COVID-19 and Future Pandemics.ACS Nano. 2024 Jan 23;18(3):1757-1777. doi: 10.1021/acsnano.3c01629. Epub 2024 Jan 8. ACS Nano. 2024. PMID: 38189684 Free PMC article. Review.
Cited by
-
Sequence-Specific Electrochemical Genosensor for Rapid Detection of blaOXA-51-like Gene in Acinetobacter baumannii.Microorganisms. 2022 Jul 13;10(7):1413. doi: 10.3390/microorganisms10071413. Microorganisms. 2022. PMID: 35889132 Free PMC article.
-
Electronic Tongue for Direct Assessment of SARS-CoV-2-Free and Infected Human Saliva-A Feasibility Study.Biosensors (Basel). 2023 Jul 7;13(7):717. doi: 10.3390/bios13070717. Biosensors (Basel). 2023. PMID: 37504115 Free PMC article.
-
Protein Stacking on the APTES-Functionalized Pyrochlore Bi2Ru2O7 Clusters for Ultrasensitive and Selective Immunosensing.ACS Appl Mater Interfaces. 2025 Feb 19;17(7):10792-10801. doi: 10.1021/acsami.4c17869. Epub 2025 Feb 6. ACS Appl Mater Interfaces. 2025. PMID: 39911050 Free PMC article.
-
Advances in Virus Biorecognition and Detection Techniques for the Surveillance and Prevention of Infectious Diseases.Biosensors (Basel). 2025 Mar 20;15(3):198. doi: 10.3390/bios15030198. Biosensors (Basel). 2025. PMID: 40136995 Free PMC article. Review.
-
Detection of SARS-CoV-2 Using Reverse Transcription Helicase Dependent Amplification and Reverse Transcription Loop-Mediated Amplification Combined with Lateral Flow Assay.Biomedicines. 2022 Sep 19;10(9):2329. doi: 10.3390/biomedicines10092329. Biomedicines. 2022. PMID: 36140431 Free PMC article.
References
-
- Huang J.C., Chang Y.-F., Chen K.-H., Su L.-C., Lee C.-W., Chen C.-C., Chen Y.-M.A., Chou C. Detection of Severe Acute Respiratory Syndrome (SARS) Coronavirus Nucleocapsid Protein in Human Serum Using a Localized Surface Plasmon Coupled Fluorescence Fiber-Optic Biosensor. Biosens. Bioelectron. 2009;25:320–325. doi: 10.1016/j.bios.2009.07.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

