A Biosensor Platform for Point-of-Care SARS-CoV-2 Screening
- PMID: 35884290
- PMCID: PMC9312522
- DOI: 10.3390/bios12070487
A Biosensor Platform for Point-of-Care SARS-CoV-2 Screening
Abstract
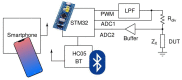
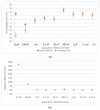
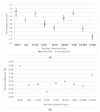
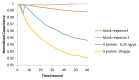
The COVID-19 pandemic remains a constant threat to human health, the economy, and social relations. Scientists around the world are constantly looking for new technological tools to deal with the pandemic. Such tools are the rapid virus detection tests, which are constantly evolving and optimizing. This paper presents a biosensor platform for the rapid detection of spike protein both in laboratory conditions and in swab samples from hospitalized patients. It is a continuation and improvement of our previous work and consists of a microcontroller-based readout circuit, which measures the capacitance change generated in an interdigitated electrode transducer by the presence either of sole spike protein or the presence of SARS-CoV-2 particles in swab samples. The circuit efficiency is calibrated by its correlation with the capacitance measurement of an LCR (inductance (L), capacitance (C), and resistance (R)) meter. The test result is made available in less than 2 min through the microcontroller's LCD (liquid-crystal display) screen, whereas at the same time, the collected data are sent wirelessly to a mobile application interface. The novelty of this research lies in the potential it offers for continuous and effective screening of SARS-CoV-2 patients, which is facilitated and enhanced, providing big data statistics of COVID-19 in terms of space and time. This device can be used by individuals for SARS-CoV-2 testing at home, by health professionals for patient monitoring, and by public health agencies for monitoring the spatio-temporal spread of the virus.
Keywords: SARS-CoV-2; interdigitated electrodes; internet of things; portable; readout; screening; smartphone; virus monitoring.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Kohmer N., Toptan T., Pallas C., Karaca O., Pfeiffer A., Westhaus S., Widera M., Berger A., Hoehl S., Kammel M., et al. Article the comparative clinical performance of four SARS-CoV-2 rapid antigen tests and their correlation to infectivity in vitro. J. Clin. Med. 2021;10:328. doi: 10.3390/jcm10020328. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

