The Potential of Novel Lipid Agents for the Treatment of Chemotherapy-Resistant Human Epithelial Ovarian Cancer
- PMID: 35884379
- PMCID: PMC9322924
- DOI: 10.3390/cancers14143318
The Potential of Novel Lipid Agents for the Treatment of Chemotherapy-Resistant Human Epithelial Ovarian Cancer
Abstract
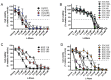
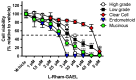
Recurrent epithelial ovarian cancer (EOC) coincident with chemotherapy resistance remains the main contributor to patient mortality. There is an ongoing investigation to enhance patient progression-free and overall survival with novel chemotherapeutic delivery, such as the utilization of antiangiogenic medications, PARP inhibitors, or immune modulators. Our preclinical studies highlight a novel tool to combat chemotherapy-resistant human EOC. Glycosylated antitumor ether lipids (GAELs) are synthetic glycerolipids capable of killing established human epithelial cell lines from a wide variety of human cancers, including EOC cell lines representative of different EOC histotypes. Importantly, GAELs kill high-grade serous ovarian cancer (HGSOC) cells isolated from the ascites of chemotherapy-sensitive and chemotherapy-resistant patients grown as monolayers of spheroid cultures. In addition, GAELs were well tolerated by experimental animals (mice) and were capable of reducing tumor burden and blocking ascites formation in an OVCAR-3 xenograft model. Overall, GAELs show great promise as adjuvant therapy for EOC patients with or without chemotherapy resistance.
Keywords: chemotherapy resistance; epithelial ovarian cancer (EOC); glycosylated antitumor ether lipid; methuosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- CCS Canadian Cancer Statistics 2021. 2021. [(accessed on 14 June 2022)]. Available online: https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2021-stat....
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Akinyemiju T.F., Al Lami F.H., Alam T., Alizadeh-Navaei R. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553–1568. doi: 10.1001/jamaoncol.2018.2706. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

