Selective CDK9 Inhibition by Natural Compound Toyocamycin in Cancer Cells
- PMID: 35884401
- PMCID: PMC9324262
- DOI: 10.3390/cancers14143340
Selective CDK9 Inhibition by Natural Compound Toyocamycin in Cancer Cells
Abstract
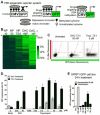
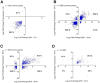
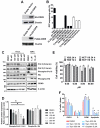
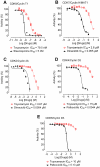
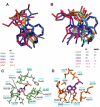
Aberrant transcription in cancer cells involves the silencing of tumor suppressor genes (TSGs) and activation of oncogenes. Transcriptomic changes are associated with epigenomic alterations such as DNA-hypermethylation, histone deacetylation, and chromatin condensation in promoter regions of silenced TSGs. To discover novel drugs that trigger TSG reactivation in cancer cells, we used a GFP-reporter system whose expression is silenced by promoter DNA hypermethylation and histone deacetylation. After screening a natural product drug library, we identified that toyocamycin, an adenosine-analog, induces potent GFP reactivation and loss of clonogenicity in human colon cancer cells. Connectivity-mapping analysis revealed that toyocamycin produces a pharmacological signature mimicking cyclin-dependent kinase (CDK) inhibitors. RNA-sequencing revealed that the toyocamycin transcriptomic signature resembles that of a specific CDK9 inhibitor (HH1). Specific inhibition of RNA Pol II phosphorylation level and kinase assays confirmed that toyocamycin specifically inhibits CDK9 (IC50 = 79 nM) with a greater efficacy than other CDKs (IC50 values between 0.67 and 15 µM). Molecular docking showed that toyocamycin efficiently binds the CDK9 catalytic site in a conformation that differs from other CDKs, explained by the binding contribution of specific amino acids within the catalytic pocket and protein backbone. Altogether, we demonstrated that toyocamycin exhibits specific CDK9 inhibition in cancer cells, highlighting its potential for cancer chemotherapy.
Keywords: CDK9 inhibitor; CDKs; RNA Pol II phosphorylation; cancer; drug screening; epigenetics; molecular docking; toyocamycin.
Conflict of interest statement
J.-P.J.I. is a founder and shareholder in Epigenonco, which seeks to develop a CDK9 inhibitor as an anti-cancer drug. The rest of the authors declare that they have no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

