Increased Soluble PD-1 Predicts Response to Nivolumab plus Ipilimumab in Melanoma
- PMID: 35884403
- PMCID: PMC9322974
- DOI: 10.3390/cancers14143342
Increased Soluble PD-1 Predicts Response to Nivolumab plus Ipilimumab in Melanoma
Abstract
Background: Checkpoint inhibitors have revolutionized the treatment of metastatic melanoma, yielding long-term survival in a considerable proportion of the patients. Yet, 40-60% of patients do not achieve a long-term benefit from such therapy, emphasizing the urgent need to identify biomarkers that can predict response to immunotherapy and guide patients for the best possible treatment. Here, we exploited an unsupervised machine learning approach to identify potential inflammatory cytokine signatures from liquid biopsies, which could predict response to immunotherapy in melanoma.
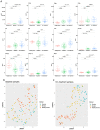
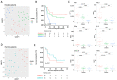
Methods: We studied a cohort of 77 patients diagnosed with unresectable advanced-stage melanoma undergoing treatment with first-line nivolumab plus ipilimumab or pembrolizumab. Baseline and on-treatment plasma samples were tested for levels of PD-1, PD-L1, IFNγ, IFNβ, CCL20, CXCL5, CXCL10, IL6, IL8, IL10, MCP1, and TNFα and analyzed by Uniform Manifold Approximation and Projection (UMAP) dimension reduction method and k-means clustering analysis.
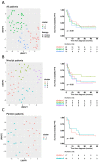
Results: Interestingly, using UMAP analysis, we found that treatment-induced cytokine changes measured as a ratio between baseline and on-treatment samples correlated significantly to progression-free survival (PFS). For patients treated with nivolumab plus ipilimumab we identified a group of patients with superior PFS that were characterized by significantly higher baseline-to-on-treatment increments of PD-1, PD-L1, IFNγ, IL10, CXCL10, and TNFα compared to patients with worse PFS. Particularly, a high PD-1 increment was a strong individual predictor for superior PFS (HR = 0.13; 95% CI 0.034-0.49; p = 0.0026). In contrast, decreasing levels of IFNγ and IL6 and increasing levels of CXCL5 were associated with superior PFS in the pembrolizumab group, although none of the cytokines were individually predictors for PFS.
Conclusions: In short, our study demonstrates that a high increment of PD-1 is associated with superior PFS in advanced-stage melanoma patients treated with nivolumab plus ipilimumab. In contrast, decreasing levels of IFNγ and IL6, and increasing levels of CXCL5 are associated with response to pembrolizumab. These results suggest that using serial samples to monitor changes in cytokine levels early during treatment is informative for treatment response.
Keywords: PD-1; immunotherapy; inflammatory cytokines; liquid biopsies; melanoma.
Conflict of interest statement
M.R.J. is shareholder and consultant for the biotech companies Stipe Therapeutics and Unikum Therapeutics who develop novel cancer immunotherapies to treat cancer. The rest of the authors have no conflicts to declare.
Figures

References
-
- Robert C., Ribas A., Schachter J., Arance A., Grob J.-J., Mortier L., Daud A., Carlino M.S., McNeil C.M., Lotem M., et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. doi: 10.1016/S1470-2045(19)30388-2. - DOI - PubMed
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022;40:127–137. doi: 10.1200/JCO.21.02229. - DOI - PMC - PubMed
-
- Schachter J., Ribas A., Long G.V., Arance A., Grob J.-J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

