Lung Nodules Missed in Initial Staging of Breast Cancer Patients in PET/MRI-Clinically Relevant?
- PMID: 35884513
- PMCID: PMC9321171
- DOI: 10.3390/cancers14143454
Lung Nodules Missed in Initial Staging of Breast Cancer Patients in PET/MRI-Clinically Relevant?
Abstract
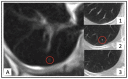
Purpose: The evaluation of the clinical relevance of missed lung nodules at initial staging of breast cancer patients in [18F]FDG-PET/MRI compared with CT.
Methods: A total of 152 patients underwent an initial whole-body [18F]FDG-PET/MRI and a thoracoabdominal CT for staging. Presence, size, shape and location for each lung nodule in [18F]FDG-PET/MRI was noted. The reference standard was established by taking initial CT and follow-up imaging into account (a two-step approach) to identify clinically-relevant lung nodules. Patient-based and lesion-based data analysis was performed.
Results: No patient with clinically-relevant lung nodules was missed on a patient-based analysis with MRI VIBE, while 1/84 females was missed with MRI HASTE (1%). Lesion-based analysis revealed 4/96 (4%, VIBE) and 8/138 (6%, HASTE) missed clinically-relevant lung nodules. The average size of missed lung nodules was 3.2 mm ± 1.2 mm (VIBE) and 3.6 mm ± 1.4 mm (HASTE) and the predominant location was in the left lower quadrant and close to the hilum.
Conclusion: All patients with newly-diagnosed breast cancer and clinically-relevant lung nodules were detected at initial [18F]FDG-PET/MRI staging. However, due to the lower sensitivity in detecting lung nodules, a small proportion of clinically-relevant lung nodules were missed. Thus, supplemental low-dose chest CT after neoadjuvant therapy should be considered for backup.
Keywords: PET/MRI; breast cancer; lung nodules; staging.
Conflict of interest statement
Christoph Rischpler reports a research grant from Pfizer; a consultancy for Adacap and Pfizer; and speaker honoraria from Adacap, Alnylam, BTG, GE Healthcare, Pfizer, and Siemens Healthineers outside of the submitted work. No other potential conflicts of interests relevant to this article exist. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Gallagher C.M., More K., Masaquel A., Kamath T., Guérin A., Ionescu-Ittu R., Nitulescu R., Gauthier-Loiselle M., Sicignano N., Butts E., et al. Survival in patients with non-metastatic breast cancer treated with adjuvant trastuzumab in clinical practice. Springerplus. 2016;5:395. doi: 10.1186/s40064-016-2008-9. - DOI - PMC - PubMed
-
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms. Version 4.4. 2021. [(accessed on 7 June 2022)]. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downlo....
Grants and funding
LinkOut - more resources
Full Text Sources

