Formulation of a Thermosensitive Imaging Hydrogel for Topical Application and Rapid Visualization of Tumor Margins in the Surgical Cavity
- PMID: 35884520
- PMCID: PMC9323389
- DOI: 10.3390/cancers14143459
Formulation of a Thermosensitive Imaging Hydrogel for Topical Application and Rapid Visualization of Tumor Margins in the Surgical Cavity
Abstract
Background: Tumor-positive surgical margins during primary breast cancer (BCa) surgery are associated with a two-fold increase in the risk of local recurrence when compared with tumor-negative margins. Pathological microscopic evaluation of the samples only assesses about 1/10 of 1% of the entire volume of the removed BCa specimens, leading to margin under-sampling and potential local recurrence in patients with pathologically clean margins, i.e., false negative margins. In the case of tumor-positive margins, patients need to undergo re-excision and/or radiation therapy, resulting in increases in complications, morbidity, and healthcare costs. Development of a simple real-time imaging technique to identify residual BCa in the surgical cavity rapidly and precisely could significantly improve the quality of care.
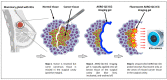
Methods: A small-molecule, fluorescently quenched protease-substrate probe, AKRO-QC-ICG, was tested as part of a thermosensitive imaging gel formulated for topical application and imaging of the BCa surgical cavity.
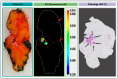
Results: More than forty formulations of gel mixtures were investigated to enable easy fluid application and subsequent solidification once applied, preventing dripping and pooling in the surgical cavity. The final formulation was tested using human BCa orthotopic implants in nude and NSG patient-derived xenografts (PDX) mice. This formulation of Pluronic F-127/DMSO/AKRO-QC-ICG imaging gel was found to be a good solvent for the probe, with a desirable thermo-reversible solid-gel transition and mechanical strength for distribution of AKRO-QC-ICG on the surfaces of tissue. It demonstrated excellent ability to detect BCa tissue after 10 min exposure, with a high signal-to-noise ratio both in mouse xenografts and freshly excised human lumpectomy tissue. The in vivo efficacy of the AKRO-QC-ICG imaging gel to detect BCa revealed the levels of sensitivity/specificity = 0.92/1 in 12 nude mice, which was corroborated with the sensitivity/specificity = 0.94/1 in 10 PDX mice.
Conclusions: Utilization of Pluronic F-127/DMSO/AKRO-QC-ICG imaging gel for topical application to detect BCa in the surgical cavity during surgery has the potential to reduce re-excisions, with consequent savings in healthcare costs and enhancement in patient quality of life.
Keywords: human breast cancer; optical imaging; surgical cavity; tumor margins.
Conflict of interest statement
Matthew Bogyo and James Basilion are co-founders, and Brian Straight is the CEO of Akrotome Imaging, Inc. (Cleveland, OH, USA) No potential conflicts of interest are disclosed by the other authors.
Figures





References
-
- American Cancer Society Breast Cancer Facts & Figures 2022. [(accessed on 11 July 2022)]. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
-
- American Cancer Society Breast Cancer Facts and Figures 2019–2020. [(accessed on 11 July 2022)]. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
-
- Moran M.S., Schnitt S.J., Giuliano A.E., Harris J.R., Khan S.A., Horton J., Klimberg S., Chavez-MacGregor M., Freedman G., Houssami N., et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann. Surg. Oncol. 2014;21:704–716. doi: 10.1245/s10434-014-3481-4. - DOI - PubMed
-
- Jeevan R., Cromwell D.A., Trivella M., Lawrence G., Kearins O., Pereira J., Sheppard C., Caddy C.M., van der Meulen J.H. Reoperation rates after breast conserving surgery for breast cancer among women in England: Retrospective study of hospital episode statistics. BMJ. 2012;345:e4505. doi: 10.1136/bmj.e4505. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

