MiRNA-Mediated Fibrosis in the Out-of-Target Heart following Partial-Body Irradiation
- PMID: 35884524
- PMCID: PMC9323333
- DOI: 10.3390/cancers14143463
MiRNA-Mediated Fibrosis in the Out-of-Target Heart following Partial-Body Irradiation
Abstract
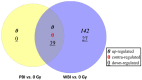
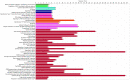
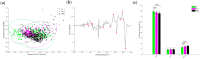
Recent reports have shown a link between radiation exposure and non-cancer diseases such as radiation-induced heart disease (RIHD). Radiation exposures are often inhomogeneous, and out-of-target effects have been studied in terms of cancer risk, but very few studies have been carried out for non-cancer diseases. Here, the role of miRNAs in the pathogenesis of RIHD was investigated. C57Bl/6J female mice were whole- (WBI) or partial-body-irradiated (PBI) with 2 Gy of X-rays or sham-irradiated (SI). In PBI exposure, the lower third of the mouse body was irradiated, while the upper two-thirds were shielded. From all groups, hearts were collected 15 days or 6 months post-irradiation. The MiRNome analysis at 15 days post-irradiation showed that miRNAs, belonging to the myomiR family, were highly differentially expressed in WBI and PBI mouse hearts compared with SI hearts. Raman spectral data collected 15 days and 6 months post-irradiation showed biochemical differences among SI, WBI and PBI mouse hearts. Fibrosis in WBI and PBI mouse hearts, indicated by the increased deposition of collagen and the overexpression of genes involved in myofibroblast activation, was found 6 months post-irradiation. Using an in vitro co-culture system, involving directly irradiated skeletal muscle and unirradiated ventricular cardiac human cells, we propose the role of miR-1/133a as mediators of the abscopal response, suggesting that miRNA-based strategies could be relevant for limiting tissue-dependent reactions in non-directly irradiated tissues.
Keywords: Raman spectroscopy; abscopal effect; cardiac fibrosis; miR-1; miRNome; mir-133a.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Milo M.L.H., Thorsen L.B.J., Johnsen S.P., Nielsen K.M., Valentin J.B., Alsner J., Offersen B.V. Risk of coronary artery disease after adjuvant radiotherapy in 29,662 early breast cancer patients: A population-based Danish Breast Cancer Group study. Radiother. Oncol. 2021;157:106–113. doi: 10.1016/j.radonc.2021.01.010. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

