Limited Benefit from the Addition of Immunotherapy to Chemotherapy in TKI-Refractory EGFR-Mutant Lung Adenocarcinoma
- PMID: 35884533
- PMCID: PMC9323840
- DOI: 10.3390/cancers14143473
Limited Benefit from the Addition of Immunotherapy to Chemotherapy in TKI-Refractory EGFR-Mutant Lung Adenocarcinoma
Abstract
Background: The benefit of chemotherapy combined with immunotherapy in EGFR-mutant lung adenocarcinoma (LUAD) patients whose tumor developed resistance to EGFR tyrosine kinase inhibitors (TKIs) is not thoroughly investigated. The goal of this retrospective cohort study is to assess the clinical efficiency of immunotherapy alone or in combination with chemotherapy in a real-world setting.
Methods: This retrospective cohort study enrolled LUAD patients with EGFR sensitive mutations whose tumor had acquired resistance to EGFR TKIs and received systemic treatment with chemotherapy (chemo; n = 84), chemotherapy combined with immunotherapy (chemoIO; n = 30), chemotherapy plus bevacizumab with or without IO (withBev; n = 42), and IO monotherapy (IO-mono; n = 22). Clinical progression-free survival (PFS) and overall survival (OS) were evaluated. Associations of clinical characteristics with outcomes were assessed using univariable and multi-covariate Cox Proportional Hazards regression models.
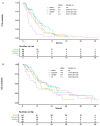
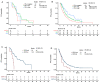
Results: A total of 178 patients (median age = 63.3; 57.9% females) with a median follow-up time of 42.0 (Interquartile range: 22.9-67.8) months were enrolled. There was no significant difference in PFS between chemoIO vs. chemo groups (5.3 vs. 4.8 months, p = 0.8). Compared to the chemo group, patients who received withBev therapy trended towards better PFS (6.1 months vs. 4.8; p = 0.3; HR 0.79; 95% CI: 0.52-1.20), while patients treated with IO-mono had inferior PFS (2.2 months; p = 0.001; HR 2.22; 95% CI: 1.37-3.59). Furthermore, PD-L1 level was not associated with PFS benefit in the chemoIO group. Patients with EGFR-mutant LUAD with high PD-L1 (≥50%) had shorter PFS (5.8 months) than non-EGFR/ALK LUAD patients who received chemoIO (12.8 months, p = 0.002; HR 0.22; 95% CI: 0.08-0.56) as first-line treatment. Chemotherapy-based therapy rendered similar benefit to patients with either EGFR exon19 deletion vs. L858R in the LUAD.
Conclusions: This retrospective analysis revealed that immunotherapy provided limited additional benefit to chemotherapy in TKI-refractory EGFR-mutant LUAD. Chemotherapy alone or combined with bevacizumab remain good choices for patients with actionable EGFR mutations.
Keywords: EGFR; chemotherapy; immunotherapy; lung adenocarcinoma; tyrosine kinase inhibitors.
Conflict of interest statement
Monique Nilsson receives royalties and licensing fees from Spectrum Pharmaceuticals, all outside of the submitted work. Marcelo Negrao reports Research funding to institution: Mirati, Novartis, Checkmate, Alaunos/Ziopharm, AstraZeneca, Pfizer, Genentech; and Consultant/Advisory Board: Mirati, Merck/MSD, Genentech, outside the submitted work. Don Gibbons reports Consulting or Advisory Role: Sanofi, GlaxoSmithKline, Janssen Research & Development, Eli Lilly, Menarini Ricerche; Research Funding: Janssen Research & Development, Takeda, AstraZeneca, Astellas, Ribon Therapeutics, NGM Biopharmaceuticals; Travel, Accommodations, Expenses: AstraZeneca/MedImmune, BerGenBio, Takeda; outside the submitted work. Natalie Vokes receives consulting fees from Sanofi Genzyme and Oncocyte, outside the submitted work. John Heymach reports receiving advisory/consulting fees from AstraZeneca, Boehringer-Ingeheim, Catalyst, Genentech, GlaxoSmithKline, Guardant Health, Foundation Medicine, Hengrui Therapeutics, Eli Lilly, Novartis, Spectrum, Sanofi, Takeda Pharmaceuticals, Mirati Therapeutics, Bristiol-Myers Squibb, BrightPath Biotherapeutics, Janssen Global Services, Nexus Health Systems, EMD Serono, Pneuma Respiratory, Kairos Venture Investments, Leads Biolabs, RefleXion, and research funding from GlaxoSmithKline, AstraZeneca, Spectrum, all outside of the submitted work. Jianjun Zhang reports grants from Merck, Novartis, Johnson and Johnson, personal fees from BMS, AZ, Novartis, Johnson and Johnson, GenePlus, Innovent, outside the submitted work. Xiuning Le reports receiving consultant and advisory fee from Eli Lilly, AstraZeneca, EMD Serono, Daiishi Sanko, Spectrum Therapeutics, Boehringer Ingelheim, Hengrui Therapeutics, Novartis, and research funding from Eli Lilly, Boehringer Ingelheim, all outside of the submitted work. Other authors declare no conflict of interest.
Figures
References
-
- Herbst R.S., Baas P., Kim D.W., Felip E., Perez-Gracia J.L., Han J.Y., Molina J., Kim J.H., Arvis C.D., Ahn M.J., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. - DOI - PubMed
-
- Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

