Genetic Polymorphisms Associated with Vincristine Pharmacokinetics and Vincristine-Induced Peripheral Neuropathy in Pediatric Oncology Patients
- PMID: 35884569
- PMCID: PMC9321338
- DOI: 10.3390/cancers14143510
Genetic Polymorphisms Associated with Vincristine Pharmacokinetics and Vincristine-Induced Peripheral Neuropathy in Pediatric Oncology Patients
Abstract
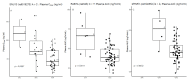
Vincristine (VCR) is an important component of curative chemotherapy for many childhood cancers. Its main side effect is VCR-induced peripheral neuropathy (VIPN), a dose limiting toxicity. Some children are more susceptible to VIPN, which is at least partially dependent on genetic factors and pharmacokinetics (PK). In this study, we identify and replicate genetic variants associated with VCR PK and VIPN. Patient samples from a randomized clinical trial studying the effect of administration duration of VCR on VIPN in 90 patients were used. PK sampling was conducted on between one and five occasions at multiple time points. A linear two-compartment model with first-order elimination was used, and targeted next-generation DNA sequencing was performed. Genotype-trait associations were analyzed using mixed-effect models or logistic regression analysis for repeated measures, or Poisson regression analysis in which the highest VIPN score per patient was included. Nine single-nucleotide polymorphisms (SNPs) in seven genes (NDRG1, GARS, FIG4, FGD4, SEPTIN9, CEP72, and ETAA1) were associated with VIPN. Furthermore, three SNPs in three genes (MTNR1B, RAB7A and SNU13) were associated with PK of VCR. In conclusion, PK of VCR and VIPN are influenced by SNPs; upfront identification of those that lead to an altered susceptibility to VIPN or VCR exposure could help individualize VCR treatment.
Keywords: DNA; area under the curve; cancer; children; maximum concentration; neurotoxicity; single-nucleotide polymorphism; toxicity; vincristine; whole-exome sequencing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Van de Velde M.E., Van den Berg M.H., Kaspers G.J.L., Abbink F.C.H., Twisk J.W.R., van der Sluis I.M., van den Bos C., van den Heuvel-Eibrink M.M., Segers H., Chantrain C., et al. The association between vincristine-induced peripheral neuropathy and health-related quality of life in children with cancer. Cancer Med. 2021;10:8172–8181. doi: 10.1002/cam4.4289. - DOI - PMC - PubMed
-
- Diouf B., Crews K.R., Lew G., Pei D., Cheng C., Bao J., Zheng J.J., Yang W., Fan Y., Wheeler H.E., et al. Association of an Inherited Genetic Variant With Vincristine-Related Peripheral Neuropathy in Children With Acute Lymphoblastic Leukemia. JAMA. 2015;313:815–823. doi: 10.1001/jama.2015.0894. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

