Multi-Targeting Approach in Glioblastoma Using Computer-Assisted Drug Discovery Tools to Overcome the Blood-Brain Barrier and Target EGFR/PI3Kp110β Signaling
- PMID: 35884571
- PMCID: PMC9317902
- DOI: 10.3390/cancers14143506
Multi-Targeting Approach in Glioblastoma Using Computer-Assisted Drug Discovery Tools to Overcome the Blood-Brain Barrier and Target EGFR/PI3Kp110β Signaling
Abstract
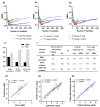
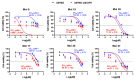
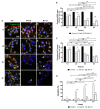
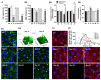
The epidermal growth factor receptor (EGFR) is upregulated in glioblastoma, becoming an attractive therapeutic target. However, activation of compensatory pathways generates inputs to downstream PI3Kp110β signaling, leading to anti-EGFR therapeutic resistance. Moreover, the blood-brain barrier (BBB) limits drugs' brain penetration. We aimed to discover EGFR/PI3Kp110β pathway inhibitors for a multi-targeting approach, with favorable ADMET and BBB-permeant properties. We used quantitative structure-activity relationship models and structure-based virtual screening, and assessed ADMET properties, to identify BBB-permeant drug candidates. Predictions were validated in in vitro models of the human BBB and BBB-glioma co-cultures. The results disclosed 27 molecules (18 EGFR, 6 PI3Kp110β, and 3 dual inhibitors) for biological validation, performed in two glioblastoma cell lines (U87MG and U87MG overexpressing EGFR). Six molecules (two EGFR, two PI3Kp110β, and two dual inhibitors) decreased cell viability by 40-99%, with the greatest effect observed for the dual inhibitors. The glioma cytotoxicity was confirmed by analysis of targets' downregulation and increased apoptosis (15-85%). Safety to BBB endothelial cells was confirmed for three of those molecules (one EGFR and two PI3Kp110β inhibitors). These molecules crossed the endothelial monolayer in the BBB in vitro model and in the BBB-glioblastoma co-culture system. These results revealed novel drug candidates for glioblastoma treatment.
Keywords: blood–brain barrier; dual-targeting; epidermal growth factor receptor; glioblastoma; phosphatidylinositol-3-kinase; quantitative structure–activity relationship models; virtual screening.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Swanson K.D., Charest A., Pollack I.F., Wong E.T. Growth Factor Signaling Pathways and Targeted Therapy. In: Newton H.B., editor. Handbook of Brain Tumor Chemotherapy, Molecular Therapeutics, and Immunotherapy. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2018. pp. 305–322.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

