Plasmacytoid Dendritic Cells, a Novel Target in Myeloid Neoplasms
- PMID: 35884612
- PMCID: PMC9317563
- DOI: 10.3390/cancers14143545
Plasmacytoid Dendritic Cells, a Novel Target in Myeloid Neoplasms
Abstract
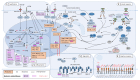
Plasmacytoid dendritic cells (pDC) are the main type I interferon producing cells in humans and are able to modulate innate and adaptive immune responses. Tumor infiltration by plasmacytoid dendritic cells is already well described and is associated with poor outcomes in cancers due to the tolerogenic activity of pDC. In hematological diseases, Blastic Plasmacytoid Dendritic Cells Neoplasm (BPDCN), aggressive leukemia derived from pDCs, is well described, but little is known about tumor infiltration by mature pDC described in Myeloid Neoplasms (MN). Recently, mature pDC proliferation (MPDCP) has been described as a differential diagnosis of BPDCN associated with acute myeloid leukemia (pDC-AML), myelodysplastic syndrome (pDC-MDS) and chronic myelomonocytic leukemia (pDC-CMML). Tumor cells are myeloid blasts and/or mature myeloid cells from related myeloid disorders and pDC derived from a clonal proliferation. The poor prognosis associated with MPDCP requires a better understanding of pDC biology, MN oncogenesis and immune response. This review provides a comprehensive overview about the biological aspects of pDCs, the description of pDC proliferation in MN, and an insight into putative therapies in pDC-AML regarding personalized medicine.
Keywords: acute myeloid leukemia; blastic plasmacytoid dendritic cells neoplasm; mature plasmacytoid dendritic cells proliferation; plasmacytoid dendritic cells; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Diagnostic approach to blastic plasmacytoid dendritic cell neoplasm: historical perspectives and current understanding.J Clin Exp Hematop. 2025;65(1):1-16. doi: 10.3960/jslrt.24069. J Clin Exp Hematop. 2025. PMID: 40159280 Free PMC article. Review.
-
Nodal Mature Plasmacytoid Dendritic Cell Proliferation in a Patient With Chronic Myelomonocytic Leukemia: A Diagnostic Mimic of Blastic Plasmacytoid Dendritic Cell Neoplasm.J Investig Med High Impact Case Rep. 2025 Jan-Dec;13:23247096251344723. doi: 10.1177/23247096251344723. Epub 2025 May 26. J Investig Med High Impact Case Rep. 2025. PMID: 40415500 Free PMC article.
-
Flow Cytometry Profiling of Plasmacytoid Dendritic Cell Neoplasms.Cancers (Basel). 2024 Jun 1;16(11):2118. doi: 10.3390/cancers16112118. Cancers (Basel). 2024. PMID: 38893237 Free PMC article. Review.
-
Plasmacytoid dendritic cell neoplasms.Blood Res. 2023 Apr 30;58(S1):90-95. doi: 10.5045/br.2023.2023052. Blood Res. 2023. PMID: 37105563 Free PMC article. Review.
-
Genetics and Epigenetics in Neoplasms with Plasmacytoid Dendritic Cells.Cancers (Basel). 2022 Aug 26;14(17):4132. doi: 10.3390/cancers14174132. Cancers (Basel). 2022. PMID: 36077669 Free PMC article. Review.
Cited by
-
Diagnostic approach to blastic plasmacytoid dendritic cell neoplasm: historical perspectives and current understanding.J Clin Exp Hematop. 2025;65(1):1-16. doi: 10.3960/jslrt.24069. J Clin Exp Hematop. 2025. PMID: 40159280 Free PMC article. Review.
-
Nodal Mature Plasmacytoid Dendritic Cell Proliferation in a Patient With Chronic Myelomonocytic Leukemia: A Diagnostic Mimic of Blastic Plasmacytoid Dendritic Cell Neoplasm.J Investig Med High Impact Case Rep. 2025 Jan-Dec;13:23247096251344723. doi: 10.1177/23247096251344723. Epub 2025 May 26. J Investig Med High Impact Case Rep. 2025. PMID: 40415500 Free PMC article.
-
Acute myeloid leukemia with plasmacytoid dendritic cell proliferation: A case report and literature review.Oncol Lett. 2025 Jul 22;30(4):456. doi: 10.3892/ol.2025.15202. eCollection 2025 Oct. Oncol Lett. 2025. PMID: 40762017 Free PMC article.
-
Clinical features and outcomes of myelodysplastic syndrome patients with iron overload: a single-center retrospective study.Eur J Med Res. 2025 Jul 9;30(1):600. doi: 10.1186/s40001-025-02848-1. Eur J Med Res. 2025. PMID: 40635108 Free PMC article.
-
Pediatric Blastic Plasmacytoid Dendritic Cell Neoplasm: A Case Report.Clin Pathol. 2024 Dec 16;17:2632010X241304564. doi: 10.1177/2632010X241304564. eCollection 2024 Jan-Dec. Clin Pathol. 2024. PMID: 39687328 Free PMC article.
References
-
- Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC Publications; Lyon, France: 2017.
-
- Zalmaï L., Viailly P.-J., Biichle S., Cheok M., Soret L., Angelot-Delettre F., Petrella T., Collonge-Rame M.-A., Seilles E., Geffroy S., et al. Plasmacytoid Dendritic Cells Proliferation Associated with Acute Myeloid Leukemia: Phenotype Profile and Mutation Landscape. Haematologica. 2021;106:3056–3066. doi: 10.3324/haematol.2020.253740. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

