Innate and Adaptive Responses of Intratumoral Immunotherapy with Endosomal Toll-Like Receptor Agonists
- PMID: 35884895
- PMCID: PMC9313389
- DOI: 10.3390/biomedicines10071590
Innate and Adaptive Responses of Intratumoral Immunotherapy with Endosomal Toll-Like Receptor Agonists
Abstract
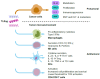
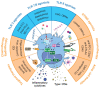
Toll-like receptors (TLRs) are natural initial triggers of innate and adaptive immune responses. With the advent of cancer immunotherapy, nucleic acids engineered as ligands of endosomal TLRs have been investigated for the treatment of solid tumors. Despite promising results, their systemic administration, similarly to other immunotherapies, raises safety issues. To overcome these problems, recent studies have applied the direct injection of endosomal TLR agonists in the tumor and/or draining lymph nodes, achieving high local drug exposure and strong antitumor response. Importantly, intratumoral delivery of TLR agonists showed powerful effects not only against the injected tumors but also often against uninjected lesions (abscopal effects), resulting in some cases in cure and antitumoral immunological memory. Herein, we describe the structure and function of TLRs and their role in the tumor microenvironment. Then, we provide our vision on the potential of intratumor versus systemic delivery or vaccination approaches using TLR agonists, also considering the use of nanoparticles to improve their targeting properties. Finally, we collect the preclinical and clinical studies applying intratumoral injection of TLR agonists as monotherapies or in combination with: (a) other TLR or STING agonists; (b) other immunotherapies; (c) radiotherapy or chemotherapy; (d) targeted therapies.
Keywords: TLR agonists; antitumoral immunotherapy; intratumoral administration; toll-like receptors; tumor associated macrophages.
Conflict of interest statement
A.C. reports a grant from Astra Zeneca and a grant from PharmaMar. The rest of the authors declare no conflict of interest.
Figures

References
-
- Mazieres J., Drilon A., Lusque A., Mhanna L., Cortot A.B., Mezquita L., Thai A.A., Mascaux C., Couraud S., Veillon R., et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann. Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

