Brain Cancer Chemotherapy through a Delivery System across the Blood-Brain Barrier into the Brain Based on Receptor-Mediated Transcytosis Using Monoclonal Antibody Conjugates
- PMID: 35884906
- PMCID: PMC9313144
- DOI: 10.3390/biomedicines10071597
Brain Cancer Chemotherapy through a Delivery System across the Blood-Brain Barrier into the Brain Based on Receptor-Mediated Transcytosis Using Monoclonal Antibody Conjugates
Abstract
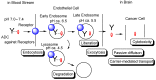
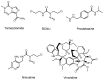
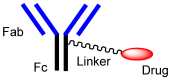
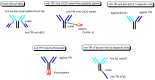
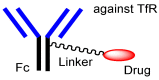
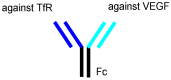
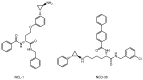
Advances in pharmacotherapy have brought extraordinary benefits to humanity. However, unmet medical needs in patients remain, particularly in the treatment of central nervous system (CNS) diseases and cancers. CNS drug delivery into the brain across the endothelium is difficult due to the blood-brain barrier (BBB), which is composed mainly of tight junctions and efflux transporters, such as multiple drug resistance 1 (MDR1) (P-glycoprotein). On the other hand, the development of anti-cancer drugs is a challenging task due to their frequent off-target side effects and the complicated mechanisms of cancer pathogenesis and progression. Brain cancer treatment options are surgery, radiation therapy, and chemotherapy. It is difficult to remove all tumor cells, even by surgical removal after a craniotomy. Accordingly, innovative brain cancer drugs are needed. Currently, antibody (Ab) drugs that show high therapeutic effects are often used clinically. Furthermore, antibody-drug conjugates (ADCs), such as trastuzumab deruxtecan, an anti-HER2 (human epidermal receptor 2) ADC with low-molecular cancer drugs through the suitable linker, have been developed. In the case of trastuzumab deruxtecan, it is internalized into cancer cells across the membrane via receptor-mediated endocytosis. Moreover, it is reported that drug delivery into the brain across the BBB was carried out via receptor-mediated transcytosis (RMT), using anti-receptor Abs as a vector against the transferrin receptor (TfR) or insulin receptor (InsR). Thus, anti-TfR ADCs with cancer drugs are promising brain cancer agents due to their precise distribution and low side effects. In this review, I introduce the implementations and potential of brain cancer drug delivery into the brain across the BBB, based on RMT using ADCs.
Keywords: anti-TfR ADCs with cancer drugs; anti-TfR and anti-EGFR bispecific ADCs with payloads; antibody-drug conjugates; brain cancer chemotherapy; drug delivery into the brain across the BBB; drug delivery system; pH-sensitive cleavable linkers; receptor-mediated transcytosis; state-of-the-art biomedicines; transferrin receptor-mediated endocytosis.
Conflict of interest statement
The author declares no conflict of interest.
Figures
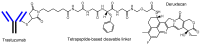



Similar articles
-
Delivery of Drugs into Cancer Cells Using Antibody-Drug Conjugates Based on Receptor-Mediated Endocytosis and the Enhanced Permeability and Retention Effect.Antibodies (Basel). 2022 Dec 19;11(4):78. doi: 10.3390/antib11040078. Antibodies (Basel). 2022. PMID: 36546903 Free PMC article. Review.
-
Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood-Brain Barrier Using Receptor-Mediated Transcytosis.Chem Pharm Bull (Tokyo). 2020;68(4):316-325. doi: 10.1248/cpb.c19-00854. Chem Pharm Bull (Tokyo). 2020. PMID: 32238649 Review.
-
Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):12486-91. doi: 10.1073/pnas.1517048112. Epub 2015 Sep 21. Proc Natl Acad Sci U S A. 2015. PMID: 26392563 Free PMC article.
-
A shorter linker in the bispecific antibody RmAb158-scFv8D3 improves TfR-mediated blood-brain barrier transcytosis in vitro.Sci Rep. 2024 Dec 23;14(1):30613. doi: 10.1038/s41598-024-83627-6. Sci Rep. 2024. PMID: 39715817 Free PMC article.
-
Receptor-mediated drug delivery of bispecific therapeutic antibodies through the blood-brain barrier.Front Drug Deliv. 2023;3:1227816. doi: 10.3389/fddev.2023.1227816. Epub 2023 Jul 10. Front Drug Deliv. 2023. PMID: 37583474 Free PMC article.
Cited by
-
HDAC Class I Inhibitor Domatinostat Preferentially Targets Glioma Stem Cells over Their Differentiated Progeny.Int J Mol Sci. 2022 Jul 22;23(15):8084. doi: 10.3390/ijms23158084. Int J Mol Sci. 2022. PMID: 35897656 Free PMC article.
-
Delivery of Drugs into Cancer Cells Using Antibody-Drug Conjugates Based on Receptor-Mediated Endocytosis and the Enhanced Permeability and Retention Effect.Antibodies (Basel). 2022 Dec 19;11(4):78. doi: 10.3390/antib11040078. Antibodies (Basel). 2022. PMID: 36546903 Free PMC article. Review.
-
State-of-the-Art Cancer Biology, Biodiagnostics and Therapeutics in Japan.Biomedicines. 2023 Oct 26;11(11):2905. doi: 10.3390/biomedicines11112905. Biomedicines. 2023. PMID: 38001906 Free PMC article.
-
Cutaneous T-cell lymphoma with CNS involvement: a case series and review of the literature.CNS Oncol. 2023 Dec 1;12(4):CNS105. doi: 10.2217/cns-2023-0014. Epub 2023 Oct 25. CNS Oncol. 2023. PMID: 37877303 Free PMC article. Review.
-
New Iron Metabolic Pathways and Chelation Targeting Strategies Affecting the Treatment of All Types and Stages of Cancer.Int J Mol Sci. 2022 Nov 13;23(22):13990. doi: 10.3390/ijms232213990. Int J Mol Sci. 2022. PMID: 36430469 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

