Inhibition of Agrobacterium tumefaciens Growth and Biofilm Formation by Tannic Acid
- PMID: 35884920
- PMCID: PMC9312696
- DOI: 10.3390/biomedicines10071619
Inhibition of Agrobacterium tumefaciens Growth and Biofilm Formation by Tannic Acid
Abstract
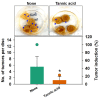
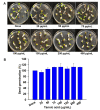
Agrobacterium tumefaciens underlies the pathogenesis of crown gall disease and is characterized by tumor-like gall formation on the stems and roots of a wide variety of economically important plant species. The bacterium initiates infection by colonizing and forming biofilms on plant surfaces, and thus, novel compounds are required to prevent its growth and biofilm formation. In this study, we investigated the ability of tannic acid, which is ubiquitously present in woody plants, to specifically inhibit the growth and biofilm formation of A. tumefaciens. Tannic acid showed antibacterial activity and significantly reduced the biofilm formation on polystyrene and on the roots of Raphanus sativus as determined by 3D bright-field and scanning electron microscopy (SEM) images. Furthermore, tannic acid dose-dependently reduced the virulence features of A. tumefaciens, which are swimming motility, exopolysaccharide production, protease production, and cell surface hydrophobicity. Transcriptional analysis of cells (Abs600 nm = 1.0) incubated with tannic acid for 24 h at 30 °C showed tannic acid most significantly downregulated the exoR gene, which is required for adhesion to surfaces. Tannic acid at 100 or 200 µg/mL limited the iron supply to A. tumefaciens and similarly reduced the biofilm formation to that performed by 0.1 mM EDTA. Notably, tannic acid did not significantly affect R. sativus germination even at 400 µg/mL. The findings of this study suggest that tannic acid has the potential to prevent growth and biofilm formation by A. tumefaciens and thus infections resulting from A. tumefaciens colonization.
Keywords: Agrobacterium tumefaciens; biofilm; quantitative reverse transcription PCR; tannic acid; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Effect of halogenated indoles on biofilm formation, virulence, and root surface colonization by Agrobacterium tumefaciens.Chemosphere. 2022 Apr;293:133603. doi: 10.1016/j.chemosphere.2022.133603. Epub 2022 Jan 12. Chemosphere. 2022. PMID: 35032513
-
Inhibition of growth, biofilm formation, virulence, and surface attachment of Agrobacterium tumefaciens by cinnamaldehyde derivatives.Front Microbiol. 2022 Oct 11;13:1001865. doi: 10.3389/fmicb.2022.1001865. eCollection 2022. Front Microbiol. 2022. PMID: 36304952 Free PMC article.
-
Virulence and biofilm inhibition of 3-methoxycinnamic acid against Agrobacterium tumefaciens.J Appl Microbiol. 2022 Nov;133(5):3161-3175. doi: 10.1111/jam.15774. Epub 2022 Aug 22. J Appl Microbiol. 2022. PMID: 35951737
-
Agrobacterium-mediated gene transfer: recent advancements and layered immunity in plants.Planta. 2022 Jul 11;256(2):37. doi: 10.1007/s00425-022-03951-x. Planta. 2022. PMID: 35819629 Free PMC article. Review.
-
Mechanisms and regulation of surface interactions and biofilm formation in Agrobacterium.Front Plant Sci. 2014 May 6;5:176. doi: 10.3389/fpls.2014.00176. eCollection 2014. Front Plant Sci. 2014. PMID: 24834068 Free PMC article. Review.
Cited by
-
A New Approach for Controlling Agrobacterium tumefaciens Post Transformation Using Lytic Bacteriophage.Plants (Basel). 2022 Nov 16;11(22):3124. doi: 10.3390/plants11223124. Plants (Basel). 2022. PMID: 36432853 Free PMC article.
-
New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition.Toxins (Basel). 2023 Sep 15;15(9):570. doi: 10.3390/toxins15090570. Toxins (Basel). 2023. PMID: 37755996 Free PMC article. Review.
-
Precision management of Fusarium fujikuroi in rice through seed coating with an enhanced nanopesticide using a tannic acid-ZnII formulation.J Nanobiotechnology. 2024 Nov 16;22(1):717. doi: 10.1186/s12951-024-02938-y. J Nanobiotechnology. 2024. PMID: 39550576 Free PMC article.
-
Anti-Agrobacterium tumefactions sesquiterpene derivatives from the marine-derived fungus Trichoderma effusum.Front Microbiol. 2024 Aug 2;15:1446283. doi: 10.3389/fmicb.2024.1446283. eCollection 2024. Front Microbiol. 2024. PMID: 39155986 Free PMC article.
-
Transcriptomic Analysis of the Negative Effect of Epigallocatechin-3-Gallate from Tea Plant (Camellia sinensis) on Agrobacterium-Mediated Transformation Efficiency.Curr Issues Mol Biol. 2025 Mar 8;47(3):178. doi: 10.3390/cimb47030178. Curr Issues Mol Biol. 2025. PMID: 40136432 Free PMC article.
References
-
- Tamzil M.S., Alfiko Y., Mubarok A.F., Purwantomo S., Suwanto A., Budiarti S. Development of Auxotrophic Agrobacterium tumefaciens AGL1 by Tn5 Transposon for Rice (Oryza sativa L.) Transformation. Biotechnol. Bioprocess Eng. 2021;26:641–649. doi: 10.1007/s12257-020-0244-x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

