Interaction Interface of Aβ42 with Human Na,K-ATPase Studied by MD and ITC and Inhibitor Screening by MD
- PMID: 35884966
- PMCID: PMC9313104
- DOI: 10.3390/biomedicines10071663
Interaction Interface of Aβ42 with Human Na,K-ATPase Studied by MD and ITC and Inhibitor Screening by MD
Abstract
Alzheimer's disease (AD) is a neurodegenerative disease accompanied by progressive cognitive and memory dysfunction due to disruption of normal electrotonic properties of neurons and neuronal loss. The Na,K-ATPase interaction with beta amyloid (Aβ) plays an important role in AD pathogenesis. It has been shown that Na,K-ATPase activity in the AD brain was significantly lower than those in age-matched control brain. The interaction of Aβ42 with Na,K-ATPase and subsequent oligomerization leads to inhibition of the enzyme activity. In this study interaction interfaces between three common Aβ42 isoforms, and different conformations of human Na,K-ATPase (α1β1) have been obtained using molecular modeling, including docking and molecular dynamics (MD). Interaction sites of Na,K-ATPase with Aβ42 are localized between extracellular parts of α- and β- subunits and are practically identical for Na,K-ATPase at different conformations. Thermodynamic parameters for the formation of Na,K-ATPase:Aβ42 complex at different conformations acquired by isothermal titration calorimetry (ITC) are similar, which is in line with the data of molecular modeling. Similarity of Na,K-ATPase interaction interfaces with Aβ in all conformations allowed us to cross-screen potential inhibitors for this interaction and find pharmaceutical compounds that could block it.
Keywords: Alzheimer’s disease; Na,K-ATPase; beta amyloid; binding constants; conformations of Na,K-ATPase; interaction inhibitors screening; interaction interface; isothermal titration calorimetry; molecular dynamics; ouabain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

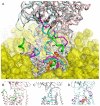

Similar articles
-
Na,K-ATPase Acts as a Beta-Amyloid Receptor Triggering Src Kinase Activation.Cells. 2022 Sep 3;11(17):2753. doi: 10.3390/cells11172753. Cells. 2022. PMID: 36078160 Free PMC article.
-
Direct interaction of beta-amyloid with Na,K-ATPase as a putative regulator of the enzyme function.Sci Rep. 2016 Jun 14;6:27738. doi: 10.1038/srep27738. Sci Rep. 2016. PMID: 27296892 Free PMC article.
-
Dysregulation of Na+/K+ ATPase by amyloid in APP+PS1 transgenic mice.BMC Neurosci. 2005 Feb 2;6:7. doi: 10.1186/1471-2202-6-7. BMC Neurosci. 2005. PMID: 15689237 Free PMC article.
-
Na⁺-K⁺-ATPase, a potent neuroprotective modulator against Alzheimer disease.Fundam Clin Pharmacol. 2013 Feb;27(1):96-103. doi: 10.1111/fcp.12000. Epub 2012 Oct 3. Fundam Clin Pharmacol. 2013. PMID: 23033963 Review.
-
[Molecular and functional diversity of NA,K-ATPase and renal H,K-ATPases].Nephrologie. 1996;17(7):401-8. Nephrologie. 1996. PMID: 9019667 Review. French.
Cited by
-
Simplified Method for Kinetic and Thermodynamic Screening of Cardiotonic Steroids through the K+-Dependent Phosphatase Activity of Na+/K+-ATPase with Chromogenic pNPP Substrate.Mol Pharmacol. 2024 Oct 17;106(5):225-239. doi: 10.1124/molpharm.124.000934. Mol Pharmacol. 2024. PMID: 39187390
-
Na,K-ATPase Acts as a Beta-Amyloid Receptor Triggering Src Kinase Activation.Cells. 2022 Sep 3;11(17):2753. doi: 10.3390/cells11172753. Cells. 2022. PMID: 36078160 Free PMC article.
-
Protein aggregation and neurodegenerative disease: Structural outlook for the novel therapeutics.Proteins. 2025 Aug;93(8):1314-1329. doi: 10.1002/prot.26561. Epub 2023 Aug 2. Proteins. 2025. PMID: 37530227 Free PMC article. Review.
-
Survey of the Aβ-peptide structural diversity: molecular dynamics approaches.Biophys Rev. 2024 Nov 20;16(6):701-722. doi: 10.1007/s12551-024-01253-y. eCollection 2024 Dec. Biophys Rev. 2024. PMID: 39830132 Review.
-
Mechanisms mediating effects of cardiotonic steroids in mammalian blood cells.Front Pharmacol. 2025 Mar 24;16:1520927. doi: 10.3389/fphar.2025.1520927. eCollection 2025. Front Pharmacol. 2025. PMID: 40196366 Free PMC article. Review.
References
-
- Karisetty B.C., Bhatnagar A., Armour E.M., Beaver M., Zhang H., Elefant F. Amyloid-β Peptide Impact on Synaptic Function and Neuroepigenetic Gene Control Reveal New Therapeutic Strategies for Alzheimer’s Disease. Front. Mol. Neurosci. 2020;13:577622. doi: 10.3389/fnmol.2020.577622. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

