Glutamate-Induced Deregulation of Krebs Cycle in Mitochondrial Encephalopathy Lactic Acidosis Syndrome Stroke-Like Episodes (MELAS) Syndrome Is Alleviated by Ketone Body Exposure
- PMID: 35884972
- PMCID: PMC9312837
- DOI: 10.3390/biomedicines10071665
Glutamate-Induced Deregulation of Krebs Cycle in Mitochondrial Encephalopathy Lactic Acidosis Syndrome Stroke-Like Episodes (MELAS) Syndrome Is Alleviated by Ketone Body Exposure
Abstract
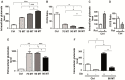
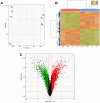
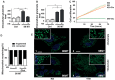
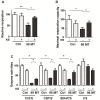
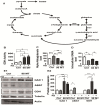
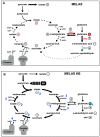
(1) Background: The development of mitochondrial medicine has been severely impeded by a lack of effective therapies. (2) Methods: To better understand Mitochondrial Encephalopathy Lactic Acidosis Syndrome Stroke-like episodes (MELAS) syndrome, neuronal cybrid cells carrying different mutation loads of the m.3243A > G mitochondrial DNA variant were analysed using a multi-omic approach. (3) Results: Specific metabolomic signatures revealed that the glutamate pathway was significantly increased in MELAS cells with a direct correlation between glutamate concentration and the m.3243A > G heteroplasmy level. Transcriptomic analysis in mutant cells further revealed alterations in specific gene clusters, including those of the glutamate, gamma-aminobutyric acid pathways, and tricarboxylic acid (TCA) cycle. These results were supported by post-mortem brain tissue analysis from a MELAS patient, confirming the glutamate dysregulation. Exposure of MELAS cells to ketone bodies significantly reduced the glutamate level and improved mitochondrial functions, reducing the accumulation of several intermediate metabolites of the TCA cycle and alleviating the NADH-redox imbalance. (4) Conclusions: Thus, a multi-omic integrated approach to MELAS cells revealed glutamate as a promising disease biomarker, while also indicating that a ketogenic diet should be tested in MELAS patients.
Keywords: MELAS syndrome; NADH/NAD imbalance; glutamate; ketone body treatment; mitochondrial diseases; mtDNA; multi-omics; tricarboxylic acid cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

