Mapping the Serum Proteome of COVID-19 Patients; Guidance for Severity Assessment
- PMID: 35884998
- PMCID: PMC9313396
- DOI: 10.3390/biomedicines10071690
Mapping the Serum Proteome of COVID-19 Patients; Guidance for Severity Assessment
Abstract
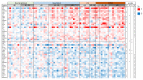
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), whose outbreak in 2019 led to an ongoing pandemic with devastating consequences for the global economy and human health. According to the World Health Organization, COVID-19 has affected more than 481 million people worldwide, with 6 million confirmed deaths. The joint efforts of the scientific community have undoubtedly increased the pace of production of COVID-19 vaccines, but there is still so much uncharted ground to cover regarding the mechanisms of SARS-CoV-2 infection, replication and host response. These issues can be approached by proteomics with unprecedented capacity paving the way for the development of more efficient strategies for patient care. In this study, we present a deep proteome analysis that has been performed on a cohort of 72 COVID-19 patients aiming to identify serum proteins assessing the dynamics of the disease at different age ranges. A panel of 53 proteins that participate in several functions such as acute-phase response and inflammation, blood coagulation, cell adhesion, complement cascade, endocytosis, immune response, oxidative stress and tissue injury, have been correlated with patient severity, suggesting a molecular basis for their clinical stratification. Eighteen protein candidates were further validated by targeted proteomics in an independent cohort of 84 patients including a group of individuals that had satisfactorily resolved SARS-CoV-2 infection. Remarkably, all protein alterations were normalized 100 days after leaving the hospital, which further supports the reliability of the selected proteins as hallmarks of COVID-19 progression and grading. The optimized protein panel may prove its value for optimal severity assessment as well as in the follow up of COVID-19 patients.
Keywords: COVID-19; SARS-CoV-2; biomarkers; proteomics; severity prognostics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

