RNA Modification in Inflammatory Bowel Diseases
- PMID: 35885000
- PMCID: PMC9313455
- DOI: 10.3390/biomedicines10071695
RNA Modification in Inflammatory Bowel Diseases
Abstract
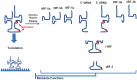
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder characterized by damage to the intestinal mucosa, which is caused by a combination of factors. These include genetic and epigenetic alterations, environmental influence, microorganism interactions, and immune conditions. Some populations with IBD show a cancer-prone phenotype. Recent studies have provided insight into the involvement of RNA modifications in the specific pathogenesis of IBD through regulation of RNA biology in epithelial and immune cells. Studies of several RNA modification-targeting reagents have shown preferable outcomes in patients with colitis. Here, we note a new awareness of RNA modification in the targeting of IBD and related diseases, which will contribute to early diagnosis, disease monitoring, and possible control by innovative therapeutic approaches.
Keywords: RNA modification; inflammatory bowel disease.
Conflict of interest statement
Partial institutional endowments were received from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan), Hirotsu Bio Science Inc. (Tokyo, Japan), Kinshu-kai Medical Corporation (Osaka, Japan), Kyowa-kai Medical Corporation (Osaka, Japan), IDEA Consultants Inc. (Tokyo, Japan), and Unitech Co., Ltd. (Chiba, Japan). G.S. is CEO of Kinshu-kai Medical Corporation. K.O. is an employee of IDEA Consultants Inc. (Tokyo, Japan). H.I. is a guest editor of the special issue of this journal.
Figures




References
-
- Bernstein C.N., Benchimol E.I., Bitton A., Murthy S.K., Nguyen G.C., Lee K., Cooke-Lauder J., Kaplan G.G. The impact of inflammatory bowel disease in Canada 2018: Extra-intestinal diseases in IBD. J. Can. Assoc. Gastroenterol. 2019;2((Suppl. 1)):S73–S80. doi: 10.1093/jcag/gwy053. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

