A Systematic Review and Meta-Analysis of Cerebrospinal Fluid Amyloid and Tau Levels Identifies Mild Cognitive Impairment Patients Progressing to Alzheimer's Disease
- PMID: 35885018
- PMCID: PMC9313367
- DOI: 10.3390/biomedicines10071713
A Systematic Review and Meta-Analysis of Cerebrospinal Fluid Amyloid and Tau Levels Identifies Mild Cognitive Impairment Patients Progressing to Alzheimer's Disease
Abstract
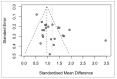
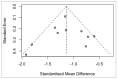
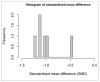
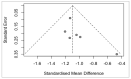
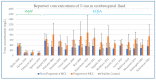
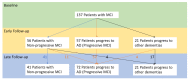
Reported levels of amyloid-beta and tau in human cerebrospinal fluid (CSF) were evaluated to discover if these biochemical markers can predict the transition from Mild Cognitive Impairment (MCI) to Alzheimer’s disease (AD). A systematic review of the literature in PubMed and Web of Science (April 2021) was performed by a single researcher to identify studies reporting immunologically-based (xMAP or ELISA) measures of CSF analytes Aβ(1-42) and/or P-tau and/or T-tau in clinical studies with at least two timepoints and a statement of diagnostic criteria. Of 1137 screened publications, 22 met the inclusion criteria for CSF Aβ(1-42) measures, 20 studies included T-tau, and 17 included P-tau. Six meta-analyses were conducted to compare the analytes for healthy controls (HC) versus progressive MCI (MCI_AD) and for non-progressive MCI (Stable_MCI) versus MCI_AD; effect sizes were determined using random effects models. The heterogeneity of effect sizes across studies was confirmed with very high significance (p < 0.0001) for all meta-analyses except HC versus MCI_AD T-tau (p < 0.05) and P-tau (non-significant). Standard mean difference (SMD) was highly significant (p < 0.0001) for all comparisons (Stable_MCI versus MCI_AD: SMD [95%-CI] Aβ(1-42) = 1.19 [0.96,1.42]; T-tau = −1.03 [−1.24,−0.82]; P-tau = −1.03 [−1.47,−0.59]; HC versus MCI_AD: SMD Aβ(1-42) = 1.73 [1.39,2.07]; T-tau = −1.13 [−1.33,−0.93]; P-tau = −1.10 [−1.23,−0.96]). The follow-up interval in longitudinal evaluations was a critical factor in clinical study design, and the Aβ(1−42)/P-tau ratio most robustly differentiated progressive from non-progressive MCI. The value of amyloid-beta and tau as markers of patient outcome are supported by these findings.
Keywords: Alzheimer’s disease; amyloid beta; biomarker; cerebrospinal fluid; meta-analysis; mild cognitive impairment; systematic review; tau.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




















References
-
- World Health Organization Life Expectancy at Birth (Years) [(accessed on 18 June 2022)]; Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/life-...
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

