Therapy Follows Diagnosis: Old and New Approaches for the Treatment of Acute Porphyrias, What We Know and What We Should Know
- PMID: 35885523
- PMCID: PMC9325038
- DOI: 10.3390/diagnostics12071618
Therapy Follows Diagnosis: Old and New Approaches for the Treatment of Acute Porphyrias, What We Know and What We Should Know
Abstract
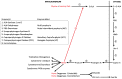
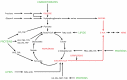
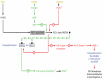
Heme, iron protoporphyrin IX, is one of life's most central molecules. Hence, availability of the enzymatic machinery necessary for its synthesis is crucial for every cell. Consequently, inborn errors of porphyrin metabolism that compromise normal synthesis, namely the family of porphyrias, undermine normal cellular metabolism given that heme has functions in catalytic centers, signal transduction and functional regulation and its synthesis is fully integrated into the center of intermediary metabolism. Very often, diagnosis of porphyrias is difficult and therefore delayed. Therapy can be as complicated. Over the last 50 years, several strategies have been developed: because of its integration with other parts of intermediary metabolism, the infusion of glucose (glucose effect) was one of the first attempts to counterbalance the dysregulation of porphyrin synthesis in porphyrias. Since heme synthesis is impaired, infusional replacement of heme was the next important therapeutic step. Recently, siRNA technology has been introduced in order to downregulate 5-ALA-synthase 1, which contributes to the patho-physiology of these diseases. Moreover, other novel therapies using enzyme protein replacement, mRNA techniques or proteostasis regulators are being developed.
Keywords: 5-ALA; acute intermittent porphyria; givosiran; glucose effect; heme; heme arginate; panhematin; variegate porphyria.
Conflict of interest statement
I have obtained honoraria for study conduct and consultation from Recordati S.p.A, Milan, Italy and Alnylam Pharmaceuticals, Cambridge, MA, USA.
Figures


References
-
- Zhang L. Heme Biology: Heme Acts as a Versatile Signaling Molecule Regulating Diverse Biological Processes. 2nd ed. World Scientific; Hackensack, NJ, USA: 2020.
-
- Taoka S., Lepore B.W., Kabil O., Ojha S., Ringe D., Banerjee R. Human cystathionine beta-synthase is a heme sensor protein. Evidence that the redox sensor is heme and not the vicinal cysteines in the CXXC motif seen in the crystal structure of the truncated enzyme. Biochemistry. 2002;41:10454–10461. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

