Type 2 Transglutaminase in Coeliac Disease: A Key Player in Pathogenesis, Diagnosis and Therapy
- PMID: 35886862
- PMCID: PMC9318967
- DOI: 10.3390/ijms23147513
Type 2 Transglutaminase in Coeliac Disease: A Key Player in Pathogenesis, Diagnosis and Therapy
Abstract
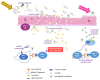
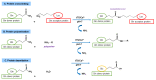
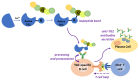
Type 2 transglutaminase (TG2) is the main autoantigen in coeliac disease (CD), a widespread inflammatory enteropathy caused by the ingestion of gluten-containing cereals in genetically predisposed individuals. As a consequence, serum antibodies to TG2 represent a very useful marker in CD diagnosis. However, TG2 is also an important player in CD pathogenesis, for its ability to deamidate some Gln residues of gluten peptides, which become more immunogenic in CD intestinal mucosa. Given the importance of TG2 enzymatic activities in CD, several studies have sought to discover specific and potent inhibitors that could be employed in new therapeutical approaches for CD, as alternatives to a lifelong gluten-free diet. In this review, we summarise all the aspects regarding TG2 involvement in CD, including its enzymatic reactions in pathogenesis, the role of anti-TG2 antibodies in disease management, and the exploration of recent strategies to reduce deamidation or to use transamidation to detoxify gluten.
Keywords: TG2 inhibitors; anti-TG2 antibodies; autoimmunity; coeliac disease; gluten; transglutaminases; type 2 transglutaminase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

