The Pro-Inflammatory Deletion Allele of the NF-κB1 Polymorphism Is Characterized by a Depletion of Subunit p50 in Sepsis
- PMID: 35886907
- PMCID: PMC9318670
- DOI: 10.3390/ijms23147559
The Pro-Inflammatory Deletion Allele of the NF-κB1 Polymorphism Is Characterized by a Depletion of Subunit p50 in Sepsis
Abstract
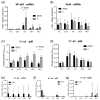
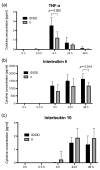
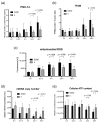
The functionally important NF-κB1 promoter polymorphism (-94ins/delATTG) significantly shapes inflammation and impacts the outcome of sepsis. However, exploratory studies elucidating the molecular link of this genotype-dependent pattern are lacking. Accordingly, we analyzed lipopolysaccharide-stimulated peripheral blood mononuclear cells from both healthy volunteers (n = 20) and septic patients (n = 10). All individuals were genotyped for the -94ins/delATTG NF-κB1 promoter polymorphism. We found a diminished nuclear activity of the NF-κB subunit p50 in ID/DD genotypes after 48 h of lipopolysaccharide stimulation compared to II genotypes (p = 0.025). This was associated with higher TNF-α (p = 0.005) and interleukin 6 concentrations (p = 0.014) and an increased production of mitochondrial radical oxygen species in ID/DD genotypes (p = 0.001). Although ID/DD genotypes showed enhanced activation of mitochondrial biogenesis, they still had a significantly diminished cellular ATP content (p = 0.046) and lower mtDNA copy numbers (p = 0.010) compared to II genotypes. Strikingly, these findings were mirrored in peripheral blood mononuclear cells taken from septic patients. Our results emphasize the crucial aspect of considering NF-κB subunits in sepsis. We showed here that the deletion allele of the NF-κB1 (-94ins/delATTG) polymorphism was associated with the lower nuclear activity of subunit p50, which, in turn, was associated with aggravated inflammation and mitochondrial dysfunction.
Keywords: NF-κB; NF-κB1 promoter polymorphism; mitochondrial dysfunction; nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells; sepsis; subunit p50.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
-
- Kellum J.A., Angus D.C. Genetic variation and risk of sepsis. Minerva Anestesiol. 2003;69:245–253. - PubMed
-
- Adamzik M., Schafer S., Frey U.H., Becker A., Kreuzer M., Winning S., Frede S., Steinmann J., Fandrey J., Zacharowski K., et al. The NFKB1 promoter polymorphism (-94ins/delATTG) alters nuclear translocation of NF-kappaB1 in monocytes after lipopolysaccharide stimulation and is associated with increased mortality in sepsis. Anesthesiology. 2013;118:123–133. doi: 10.1097/ALN.0b013e318277a652. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

